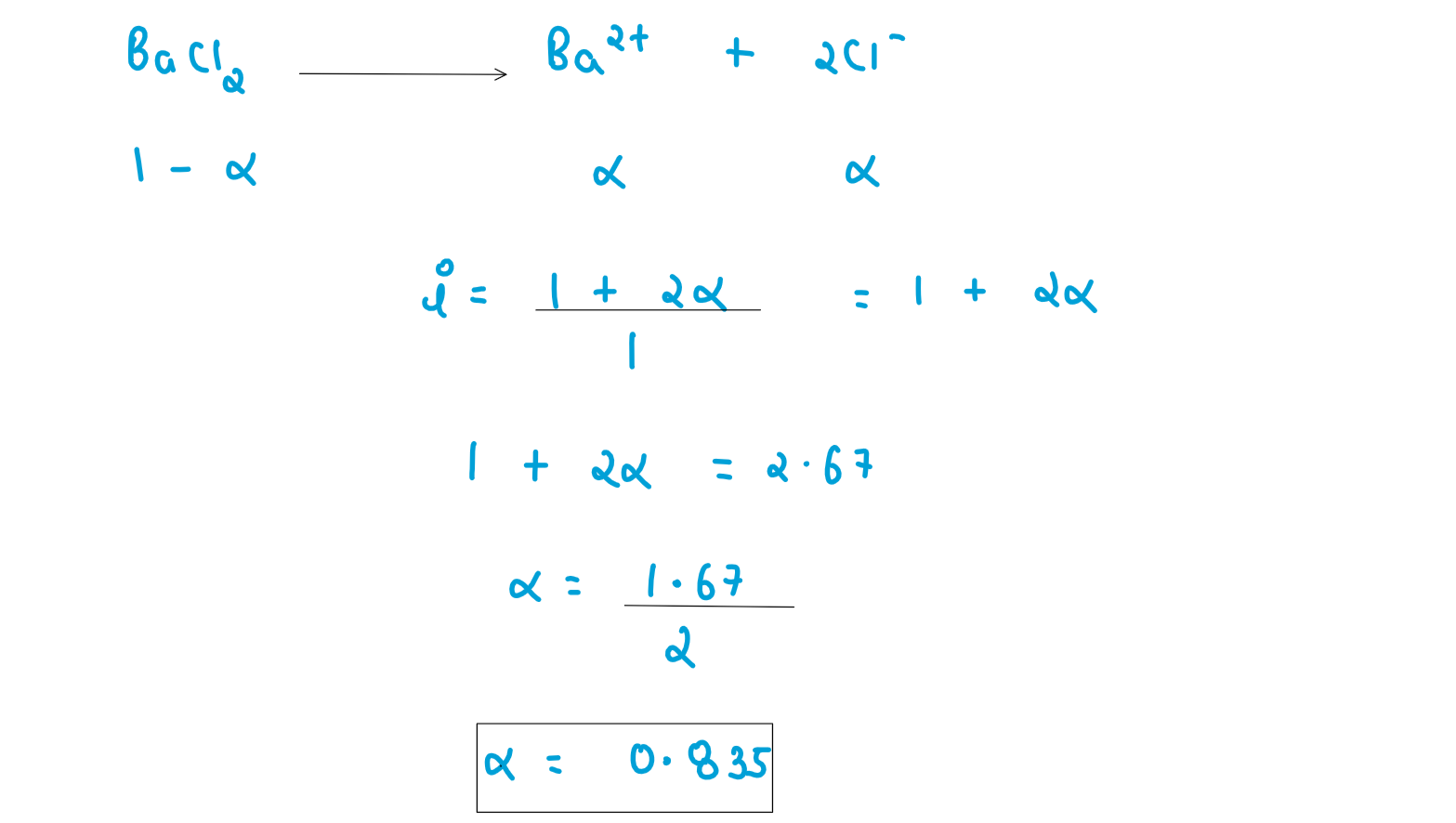An aqueous solution containing 12.50 g of barium chloride in 1000g of water boils at 373.0834 K. Calculate the degree of dissociation of barium chloride. Given KB for H20 = 0.52 K kg mol-1 molecular mass of BaCl2 = 208.34 g mol-1
Solution
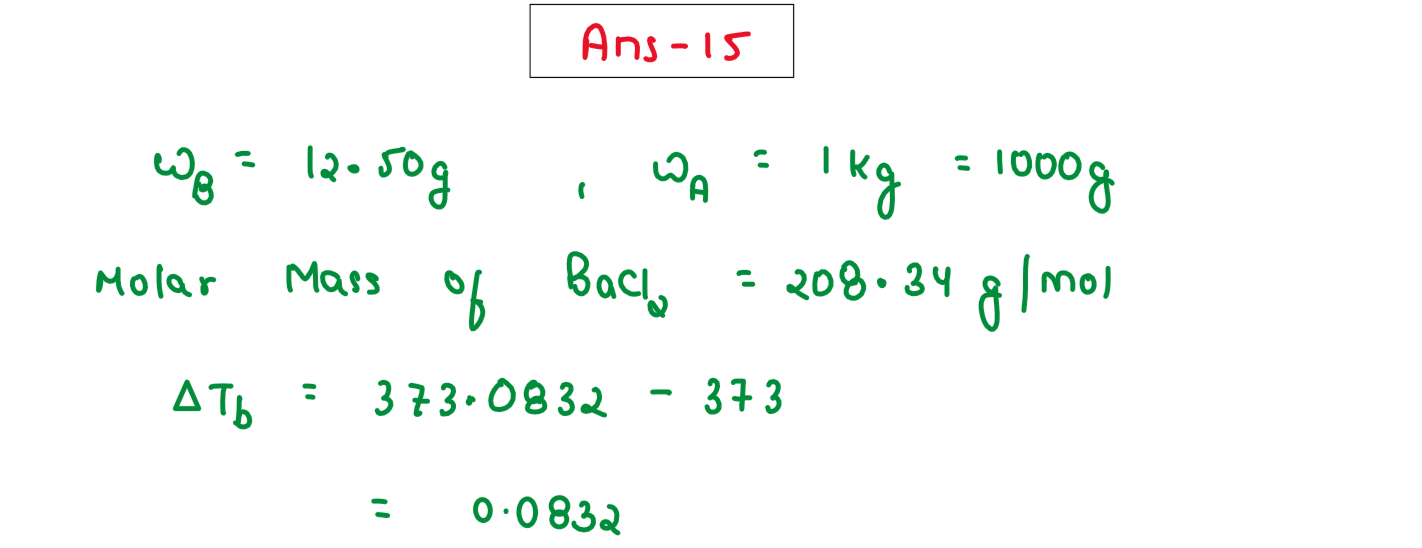 ,
,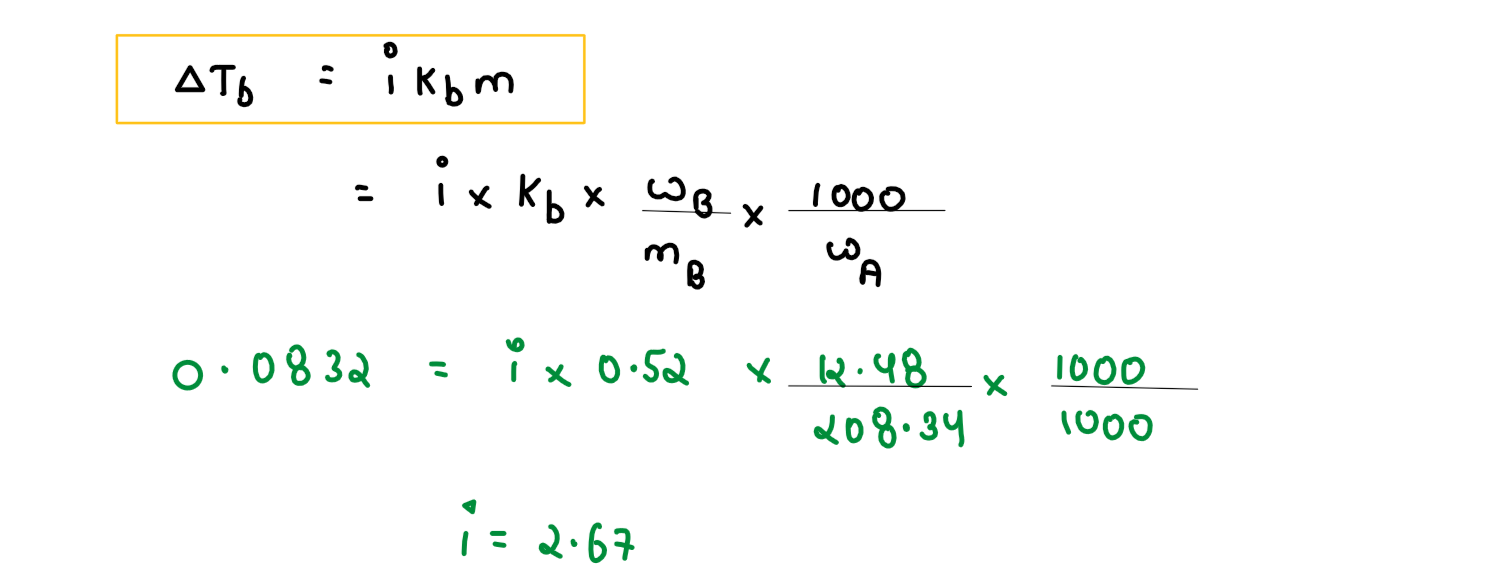 ,
,