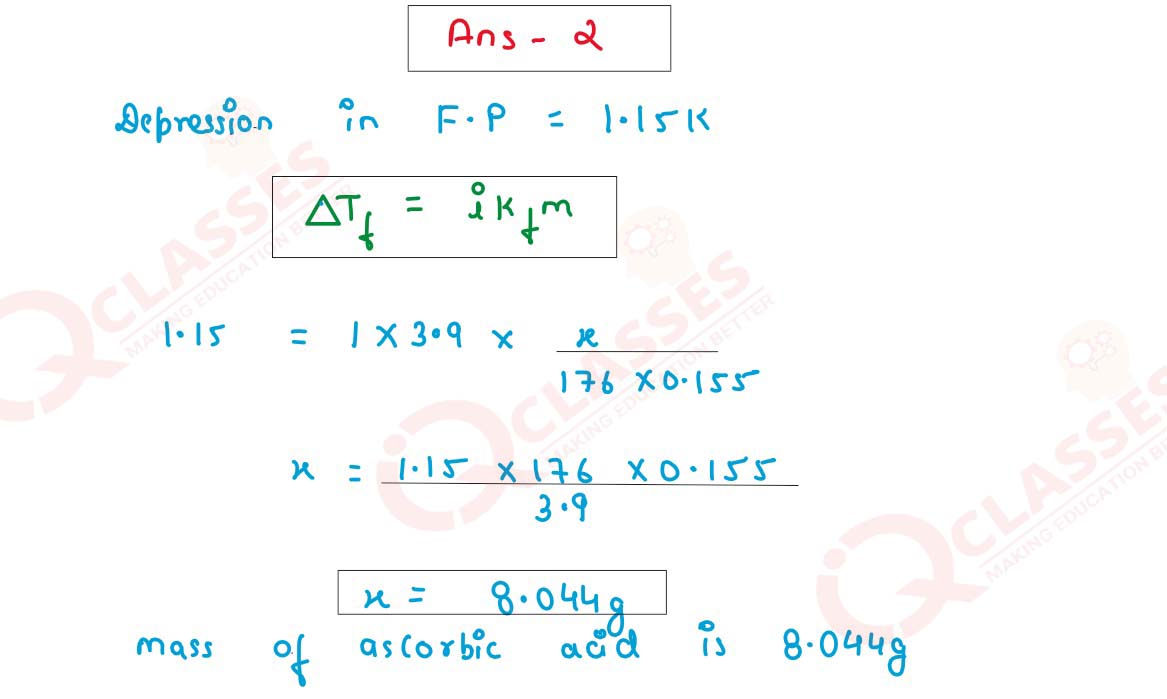
Calculate the mass of ascorbic acid (molecular mass = 176 g/mol) that should be dissolved in 155g of acetic acid to cause a depression of freezing point by 1.15K. Assume that ascorbic acid does not dissociate or associate in the solution.
(Kf for acetic acid = 3.9K kg/mol)
Solution