Class 10 ICSE Chemistry Specimen 2023
Maximum Marks: 80
Time allowed: Two and half hours
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during first 15 minutes.
This time is to be spent in reading the question paper.
The time given at the head of this Paper is the time allowed for writing the answers.
Section A is compulsory.
Attempt any four questions from Section B.
The intended marks for questions or parts of questions are given in brackets [ ].
Section-A
(Attempt all questions from this Section.)
Question 1
Choose one correct answer to the questions from the given options:
(i) A weak electrolyte is:
(a) Alcohol
(b) Potassium hydroxide
(c) Ammonium hydroxide
(d) Glucose
Solution
(c) Ammonium hydroxide
(ii) Electron affinity is maximum in:
(a) Alkaline earth metals
(b) Halogens
(c) Inert gases
(d) Alkali metals
Solution
(b) Halogens
(iii) The main components of bronze are:
(a) Copper and tin
(b) Copper and iron
(c) Copper and lead
(d) Copper and zinc
Solution
(a) Copper and tin
(iv) A polar covalent compound is:
(a) Methane
(b) Ammonia
(c) Nitrogen
(d) Chlorine
Solution
(b) Ammonia
(v) An acid which has two replaceable hydrogen ions:
(a) Acetic acid
(b) Hydrochloric acid
(c) Phosphoric acid
(d) Carbonic acid
Solution
(d) Carbonic acid
(vi) The hydroxide which is soluble in excess of NaOH is:
(a) Ferric hydroxide
(b) Lead hydroxide
(c) Copper hydroxide
(d) Calcium hydroxide
Solution
(b) Lead hydroxide
(vii) If the RMM of carbon dioxide is 44, then its vapour density is:
(a) 22
(b) 32
(c) 44
(d) 88
Solution
(a) 22
(viii) Drying agent used to dry Hydrogen chloride gas:
(a) Concentrated Sulphuric acid
(b) Calcium oxide
(c) Sulphurous acid
(d) Calcium hydroxide
Solution
(a) Concentrated Sulphuric acid
(ix) The catalyst used in the Haber’s Process is:
(a) Molybdenum
(b) Platinum
(c) Nickel
(d) Finely divided Iron
Solution
(d) Finely divided Iron
(x) An aqueous compound which turns colourless phenolphthalein to pink:
(a) Ammonium hydroxide
(b) Nitric acid
(c) Anhydrous calcium chloride
(d) Sulphuric acid
Solution
(a) Ammonium hydroxide
(xi) The gas formed when carbon reacts with concentrated sulphuric acid:
(a) Hydrogen
(b) Sulphur trioxide
(c) Sulphur dioxide
(d) Oxygen
Solution
(c) Sulphur dioxide
(xii) The organic compound prepared when Ethanol undergoes dehydration:
(a) Methane
(b) Ethane
(c) Acetylene
(d) Ethene
Solution
(d) Ethene
(xiii) The IUPAC name of methyl acetylene ts:
(a) Propyne
(b) Ethene
(c) Propane
(d) Ethyne
Solution
(a) Propyne
(xiv) The product formed at the cathode in electroplating of an article with Nickel is:
(a) Hydrogen gas
(b) Nickel ions
(c) Nickel atoms
(d) Oxygen gas
Solution
(c) Nickel atoms
(xv) An alkali metal found in period 3 and group 1 is:
(a) Magnesium
(b) Lithium
(c) Sodium
(d) Potassium
Solution
(c) Sodium
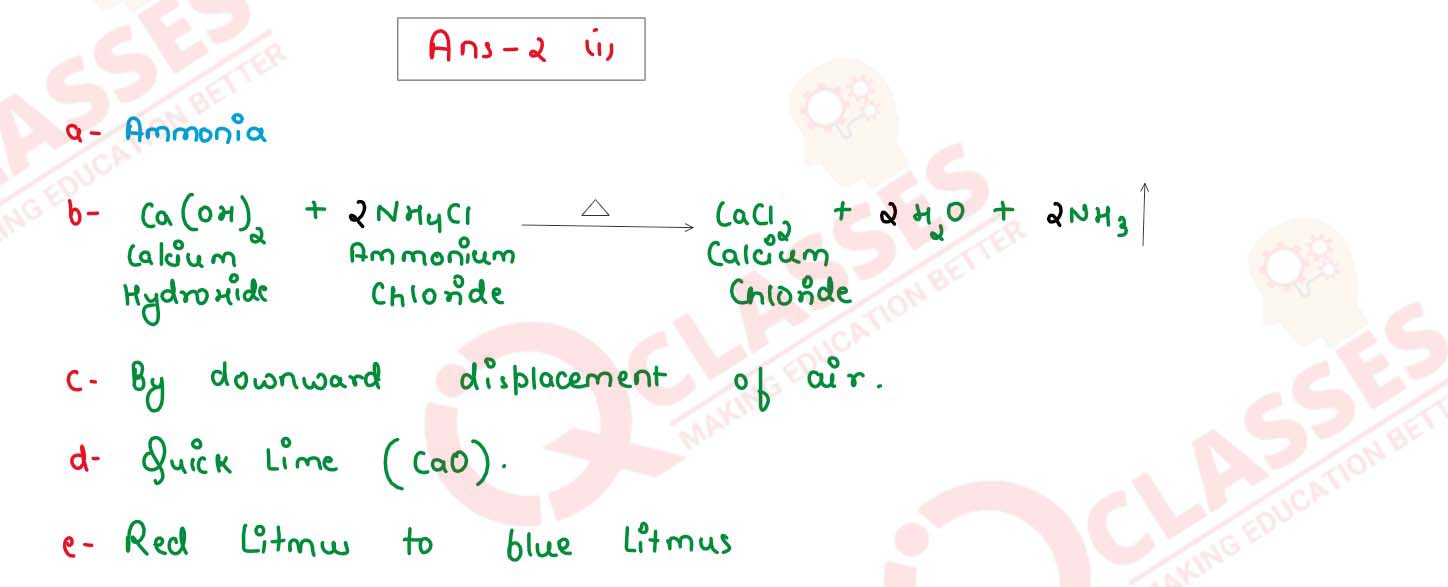
Question 2
(i) The diagram shows an experiment set up for the laboratory preparation of a
pungent
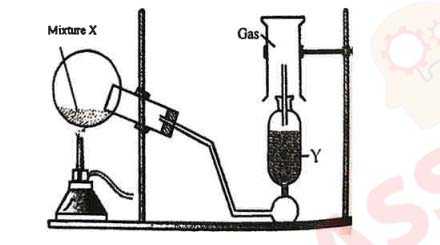
smelling gas. The gas is alkaline in nature.
(a) Name the gas collected in the gas jar.
(b) Write a balanced chemical equation for the above preparation.
(c) How is the gas being collected?
(d) What is the purpose of using Y?
(e)
How will you find that the jar is full of gas?
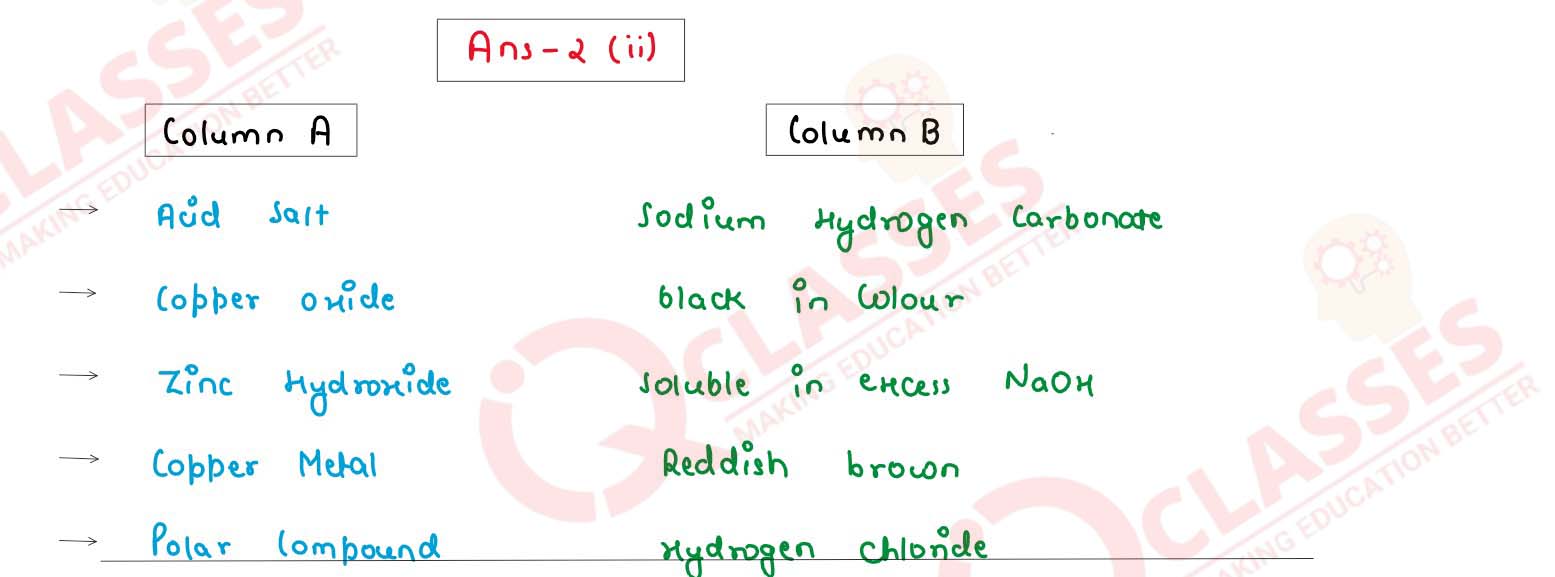
(ii) Match the following Column A with Column B.

(iii) Complete the following by choosing the correct answers from the bracket:
(a) Ammonia in the liquefied form is........ [neutral / basic]
(b) Organic compounds are generally insoluble in ......... [Water / Organic
solvents]
(c) An inert electrode used in electrolysis of acidified water is...................
[iron / platinum]
(d) Hydrocarbons having double bond is .......... [alkenes / alkynes]
(e) Analkaline gas gives dense white fumes of.......... [NH4OH / NHaCl] with
hydrogen chloride gas.
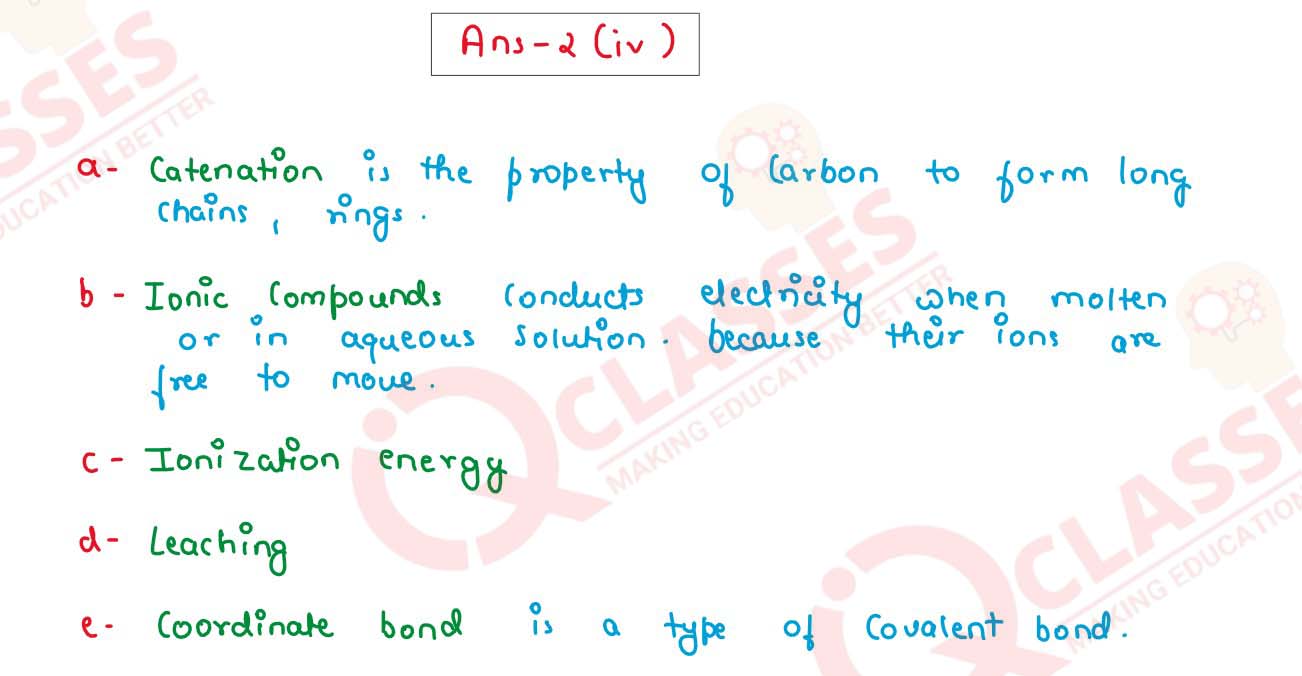
(iv) Identify the following:
(a) The property by which carbon bonds with itself to form a long chain.
(b) A substance that conducts electricity in molten or aqueous state.
(c) The energy required to remove an electron from the valence shell of a neutral
isolated gaseous atom.
(d) The name of the process by which the Bauxite ore is concentrated.
(e) The bond formed by a shared pair of electrons with both electrons coming from
the same atom.
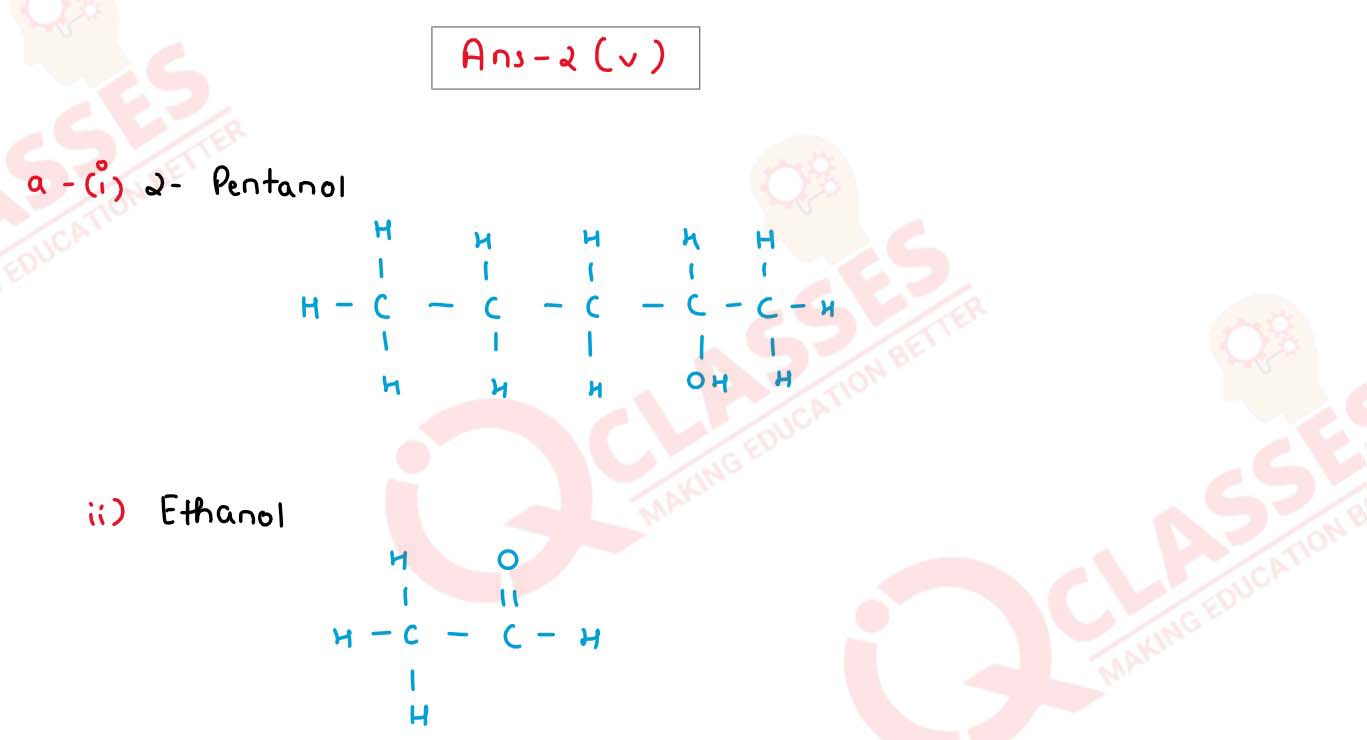
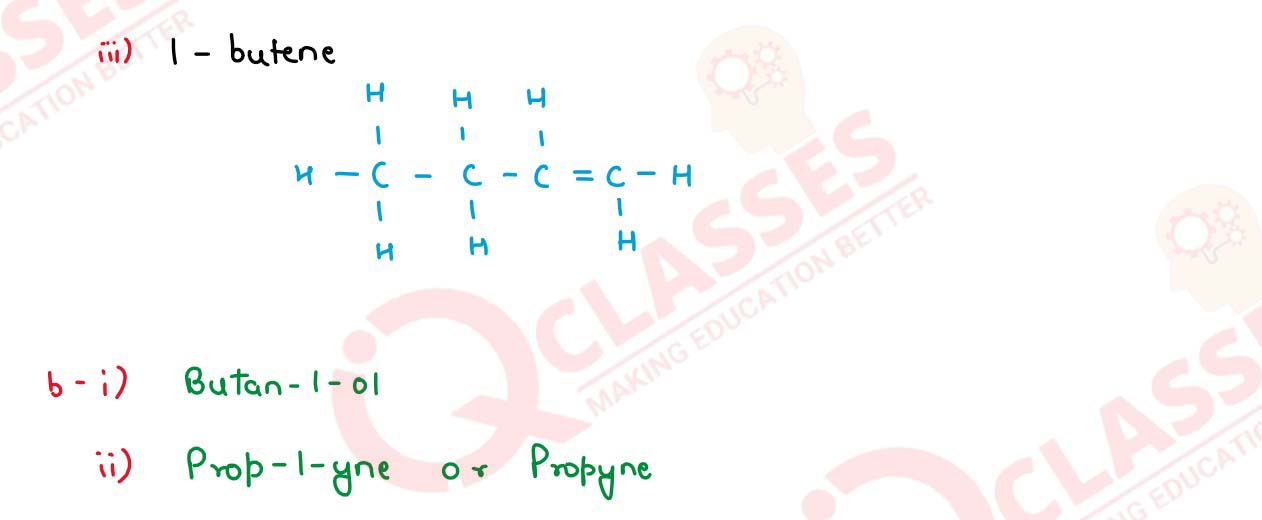
(v) (a) Draw the structural formula for the following:
1. 2-pentanol
2. Ethanal
3. 1-butene
(b) Name the following organic compounds in IUPAC system:
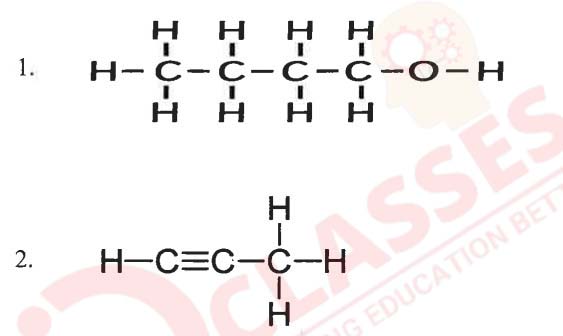
Solution

Section-B
(Attempt any four questions from this Section.)
Question 3
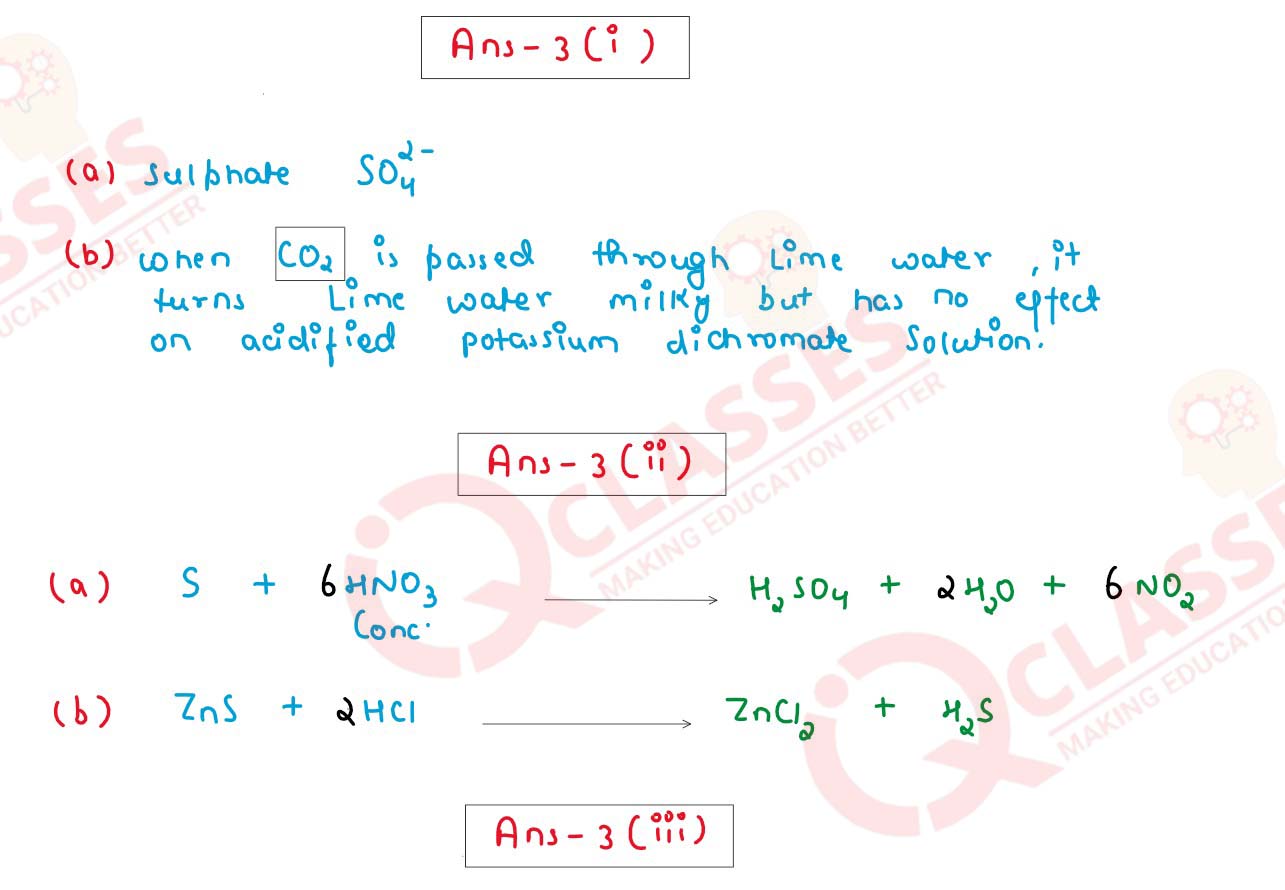
(i) Identify the Anion present in each of the following compounds.
(a) When Barium Chloride Solution is added to a solution of compound B, a white
precipitate insoluble in dilute Hydrochloric acid is formed.
(b) When dilute Sulphuric acid is added to compound D, a gas is produced which
turns lime water milky but has no effect on acidified potassium dichromate
solution.
(ii) Write the products and balance the equation.
(a) S + Conc HNO3 →
(b) ZnS + HCl →
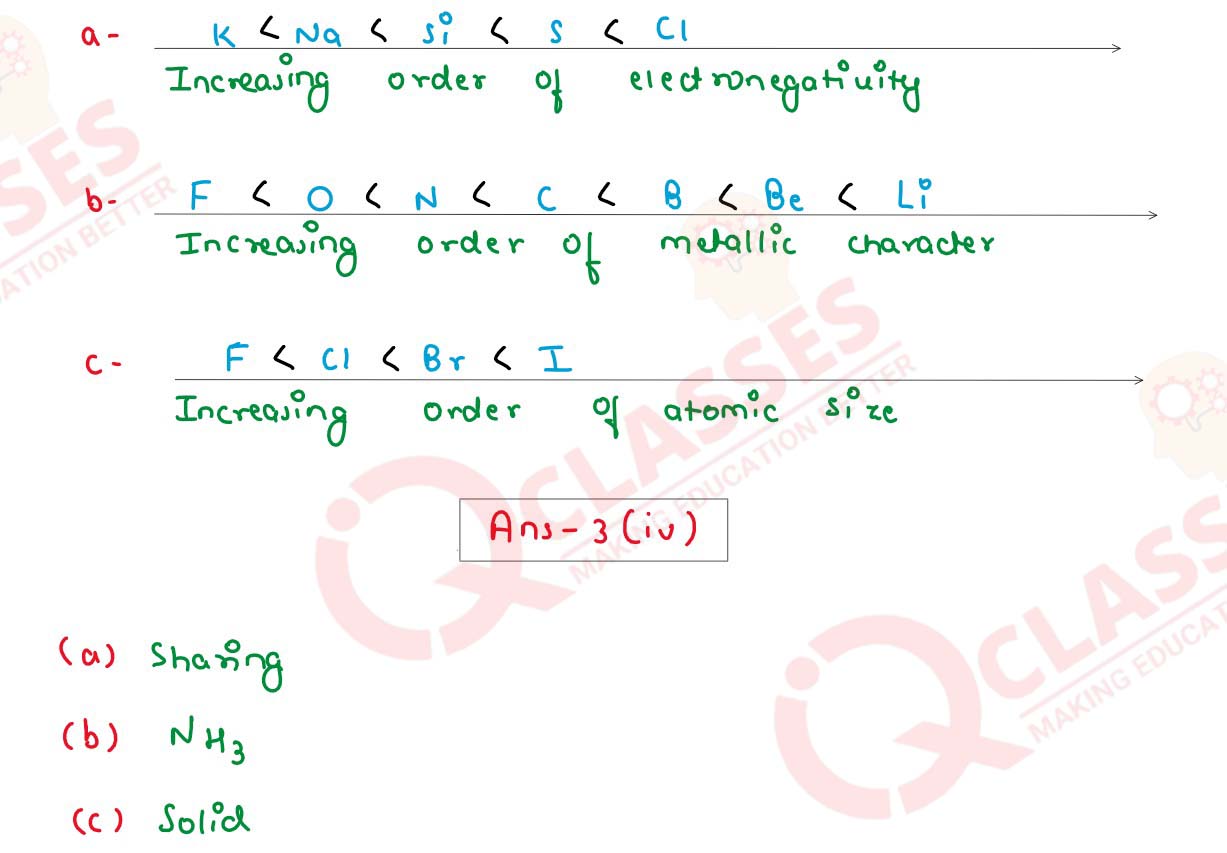
(iii) Arrange the following as per the instruction given in the brackets:
(a) Na,K, Cl, Si, S. (increasing order of electro negativity)
(b) Be, Li, F,C,B,N,O (increasing order of metallic character)
(c) Br, F,I, Cl (increasing order of atomic size)
(iv) Fill in the blanks selecting the appropriate word from the given choice:
(a) In a covalent compound the bond is formed due to of electrons ..............
(sharing / transfer)
(b) A molecule which has a single lone pair of electrons ......... (NH3 / H20)
(c) Electrovalent compounds do not conduct electricity in their state.............
(molten / solid)
Solution
Section-B
(Attempt any four questions from this Section.)
Question 4
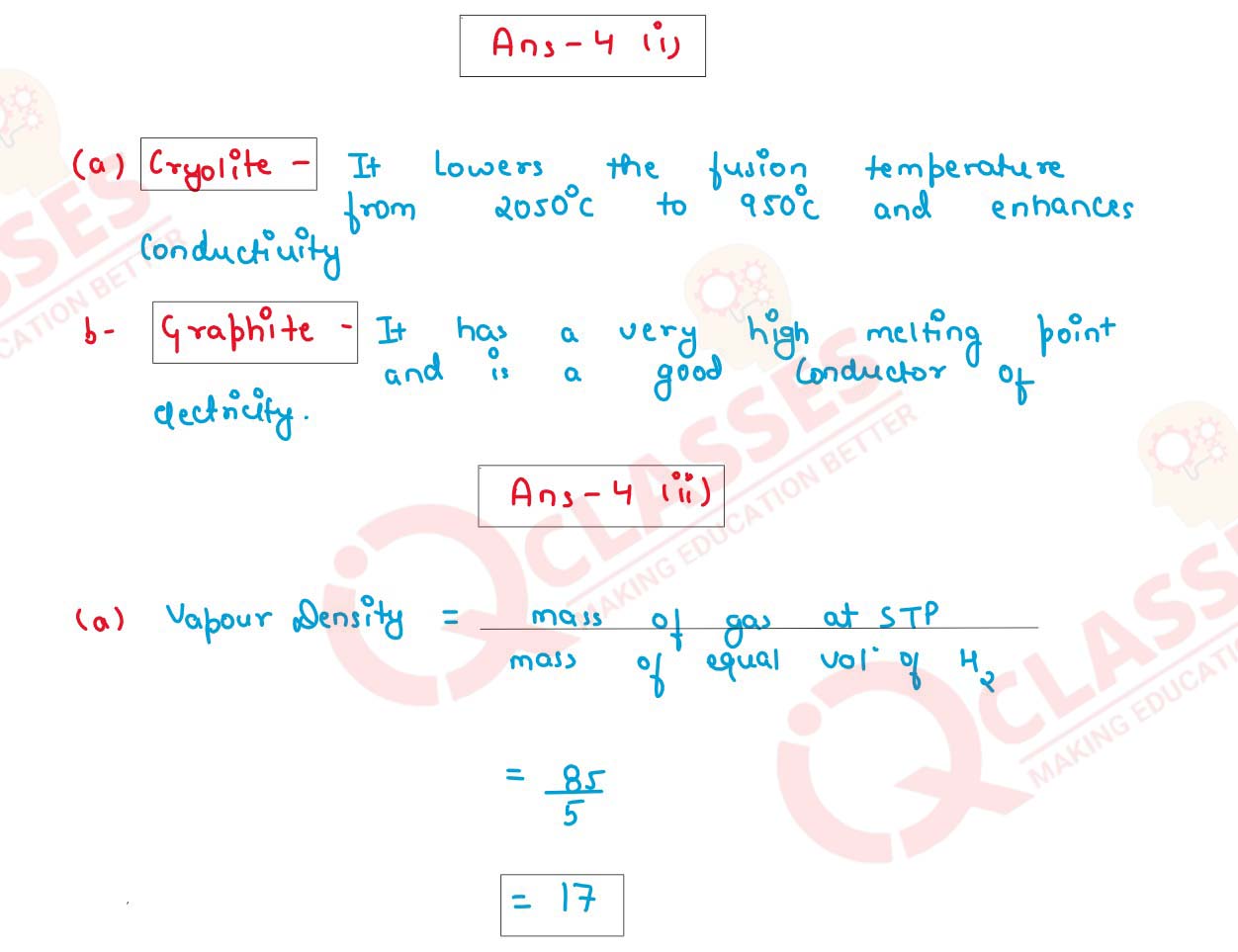
(i) For each of the substances given below, what is the role played in the extraction of
Aluminum.
(a) Cryolite
(b) Graphite
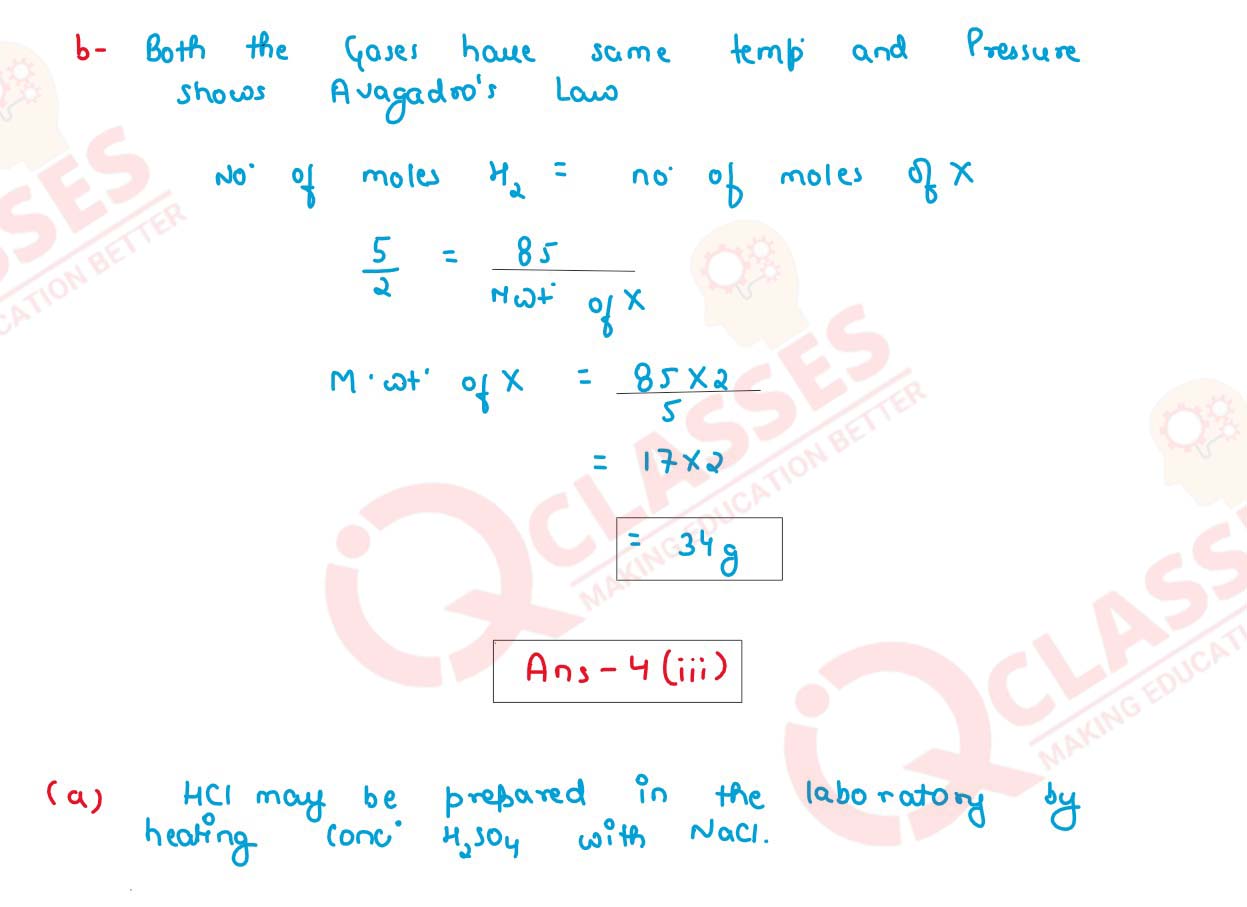
(ii) Calculate:
(a) A gas cylinder is filled with hydrogen and it holds 5 gms of gas. The same
cylinder holds 85 gms of gas X under same temperature and pressure.
Calculate the vapour density of gas X.
(b) Give the empirical formula of CH3COOH.
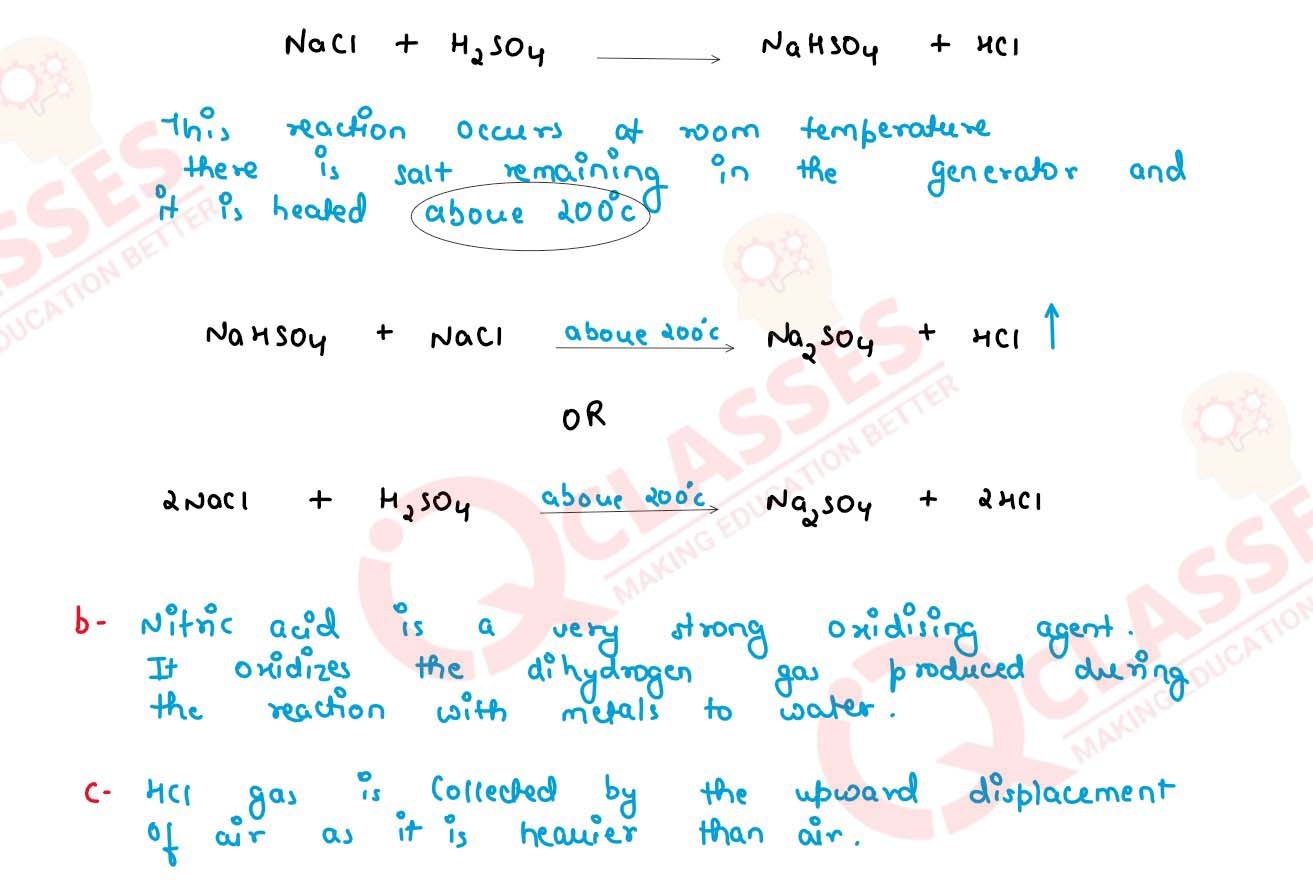
(iii) The following questions are pertaining to the laboratory preparation of Hydrogen
chloride gas.
(a) Write a balanced chemical equation for its preparation mentioning the
condition required.
(b) Why is concentrated Nitric Acid not used in the preparation of Hydrogen
Chloride gas?
(c) How is Hydrogen Chloride gas collected?
(iv) Explain the following:
(a) Concentrated Nitric acid appears yellow when it is left standing in a glass
bottle.
(b) An inverted Funnel is used to dissolve Hydrogen Chloride gas in water.
(c) All apparatus made of glass is used in the laboratory preparation of Nitric acid.
Solution

Question 5
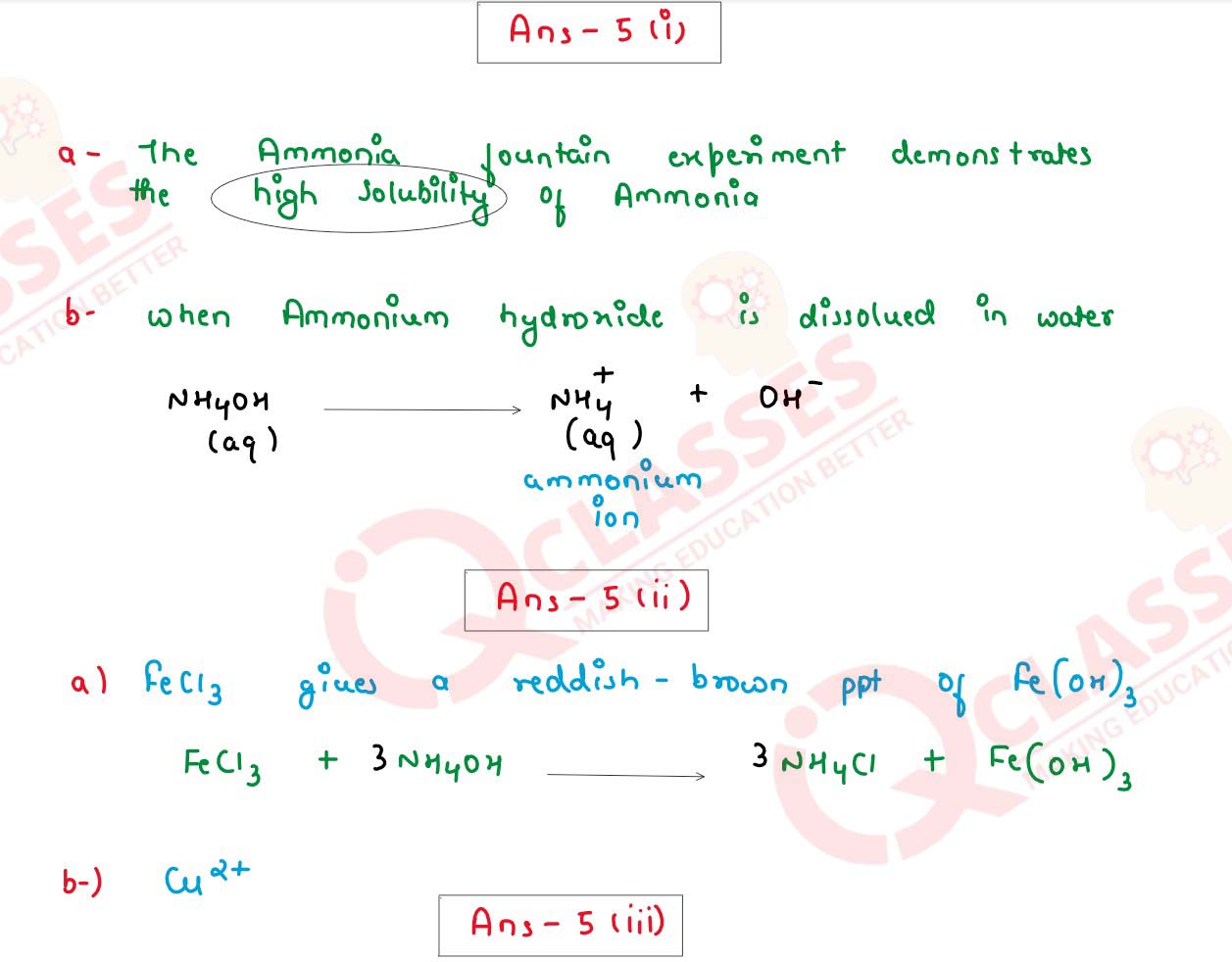
(i) (a) State one property of Ammonia demonstrated in the Fountain Experiment.
(b) Give the ionic equation when Ammonium Hydroxide is dissolved in water.
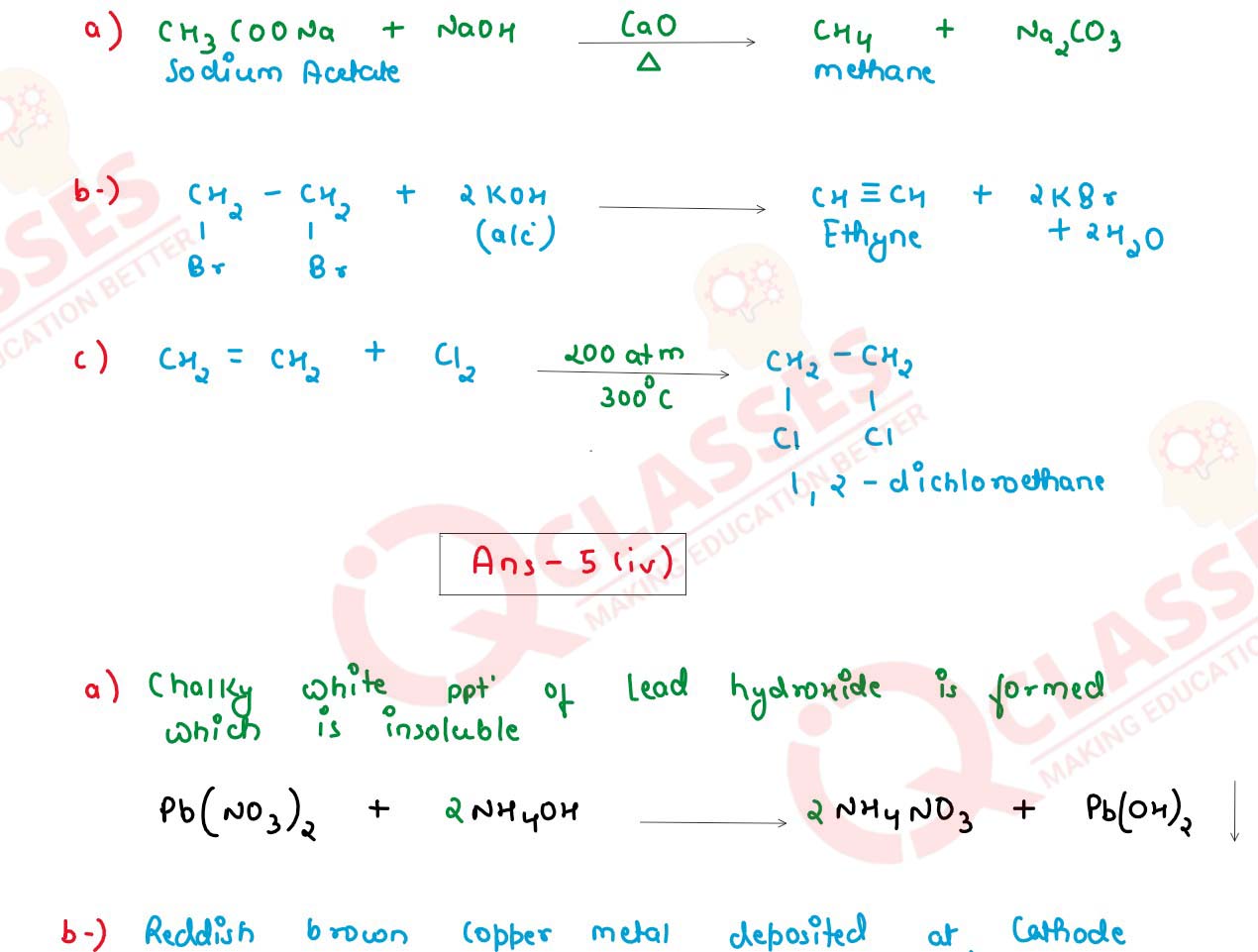
(ii) Name a probable Cation present based on the following Observations:
(a) Reddish brown precipitate insoluble in Ammonium Hydroxide.
(b) Blue coloured sulphate solution.
(iii) Give balanced chemical equation for the following:
(a) Laboratory Preparation of Methane from Sodium Acetate.
(b) Preparation of Ethyne from 1, 2 dibromoethane.
(c) Ethene reacting with Chlorine.
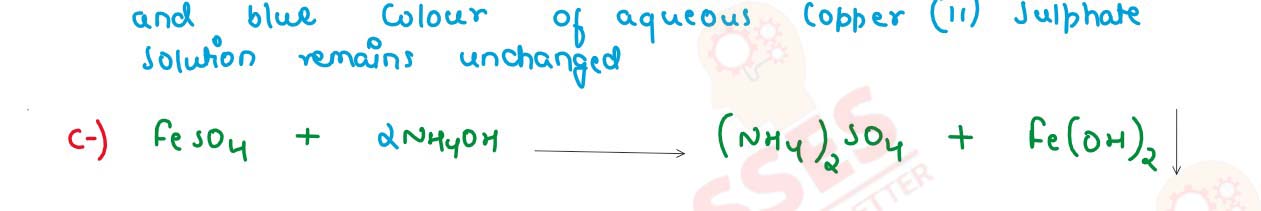
(iv) State one relevant observation for each of the following reactions:
(a) When excess Ammonia is passed through an aqueous solution of Lead Nitrate.
(b) Copper Sulphate solution is electrolysed using Copper electrodes.
(c) Ammonium hydroxide is added to Ferrous Sulphate solution.
Solution
Question 6
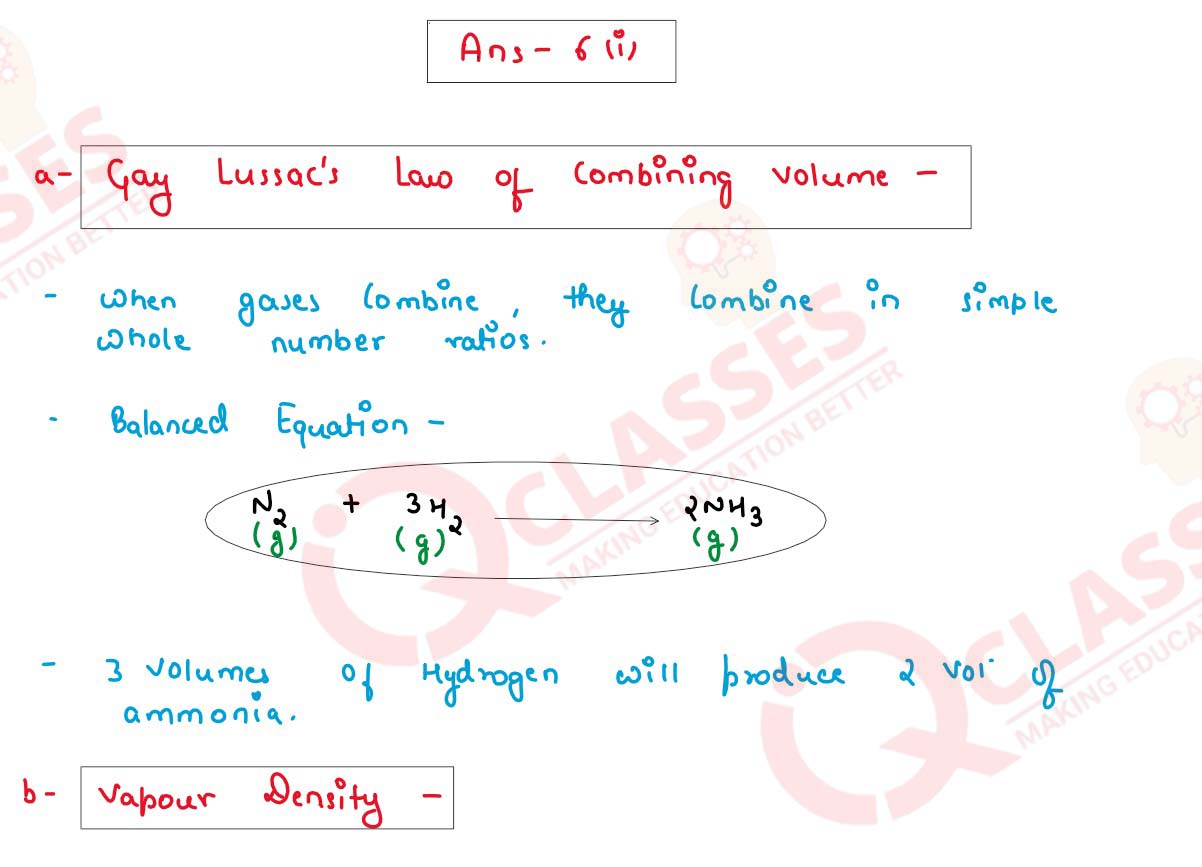
(i) Define:
(a) Gay Lussac’s law of combining volume.
(b) Vapour Density
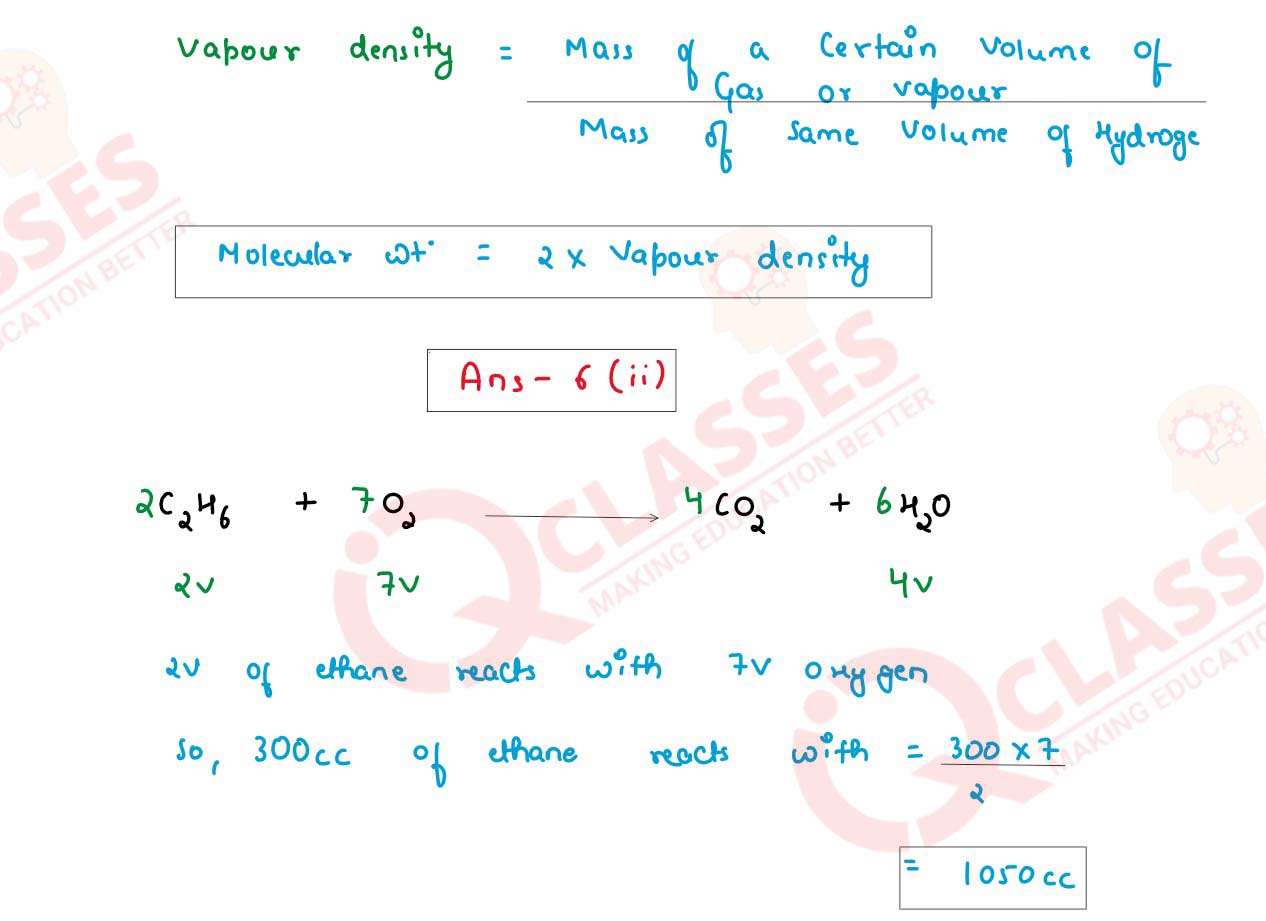
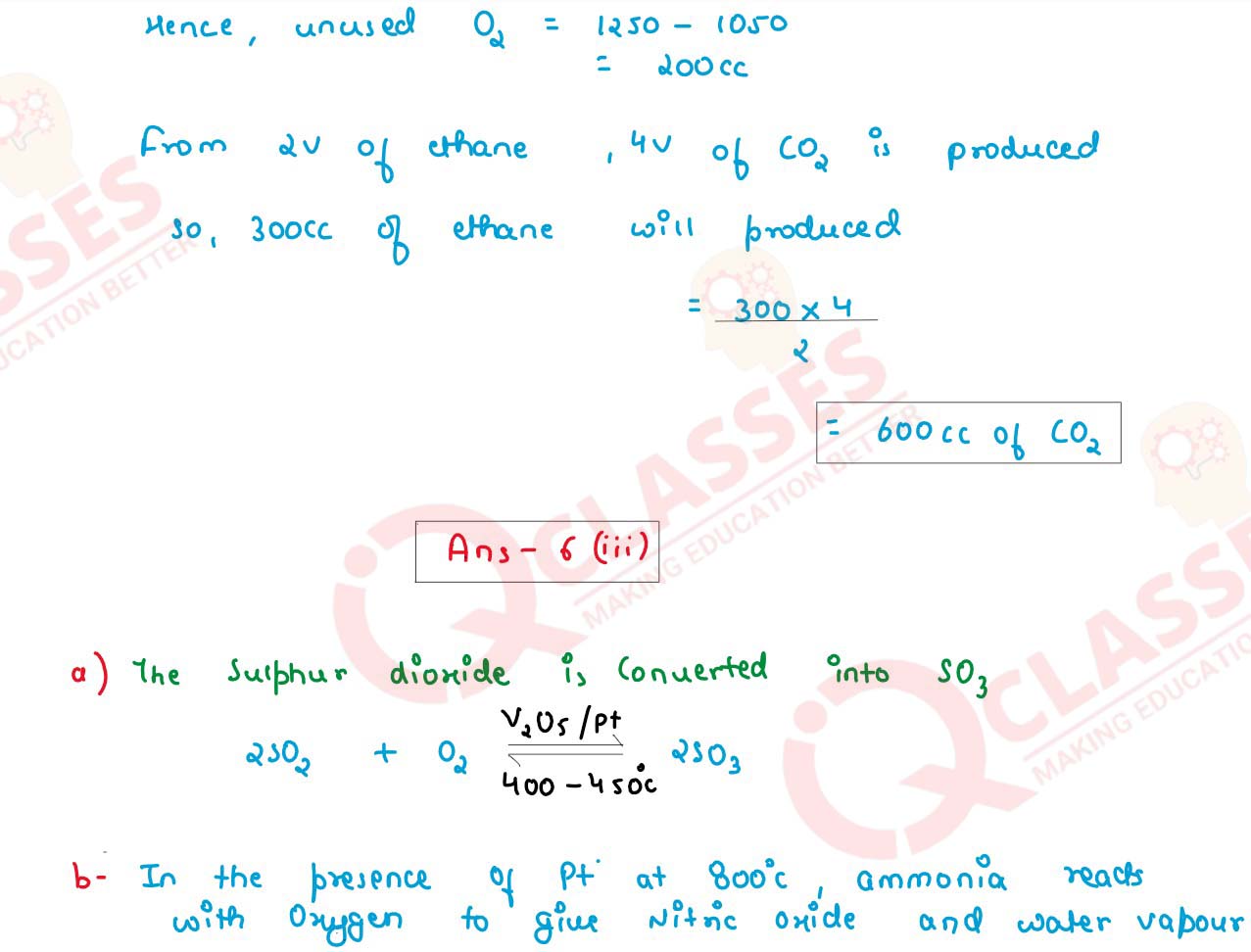
(ii) Solve:
1250cc of oxygen was burnt with 300cc of ethane (C2H6). Calculate the volume of
the unused oxygen.and the volume of the carbon dioxide formed.
2C2H6 + 702 → 4CO2 + 6H20
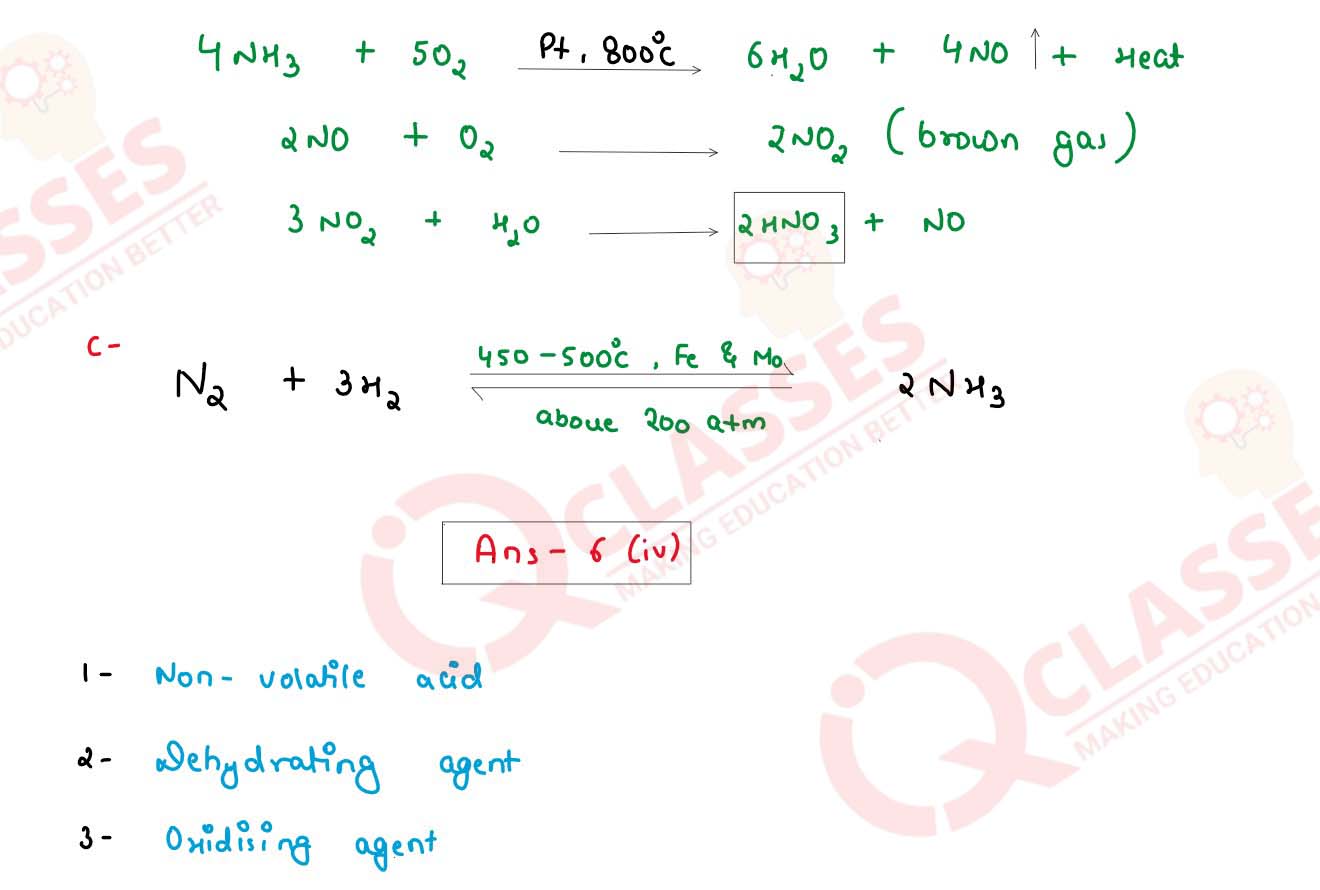
(iii) State the conditions required for the following reactions:
(a) Conversion of Sulphur dioxide to Sulphur trioxide.
(b) Conversion of Ammonia to Nitric acid
(c) Conversion of Nitrogen to Ammonia
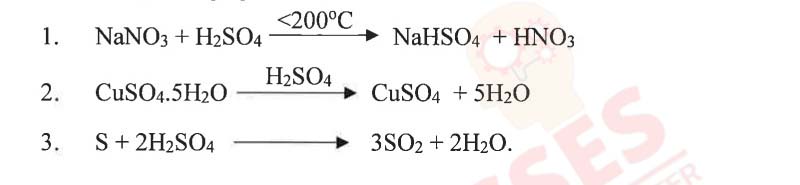
(iv) Choose the role played by concentrated Sulphuric acid as A, B, C which is
responsible for the reactions 1 to 3.
A. Oxidizing agent
B. Non Volatile Acid
C. Dehydrating agent
Solution
Question 7
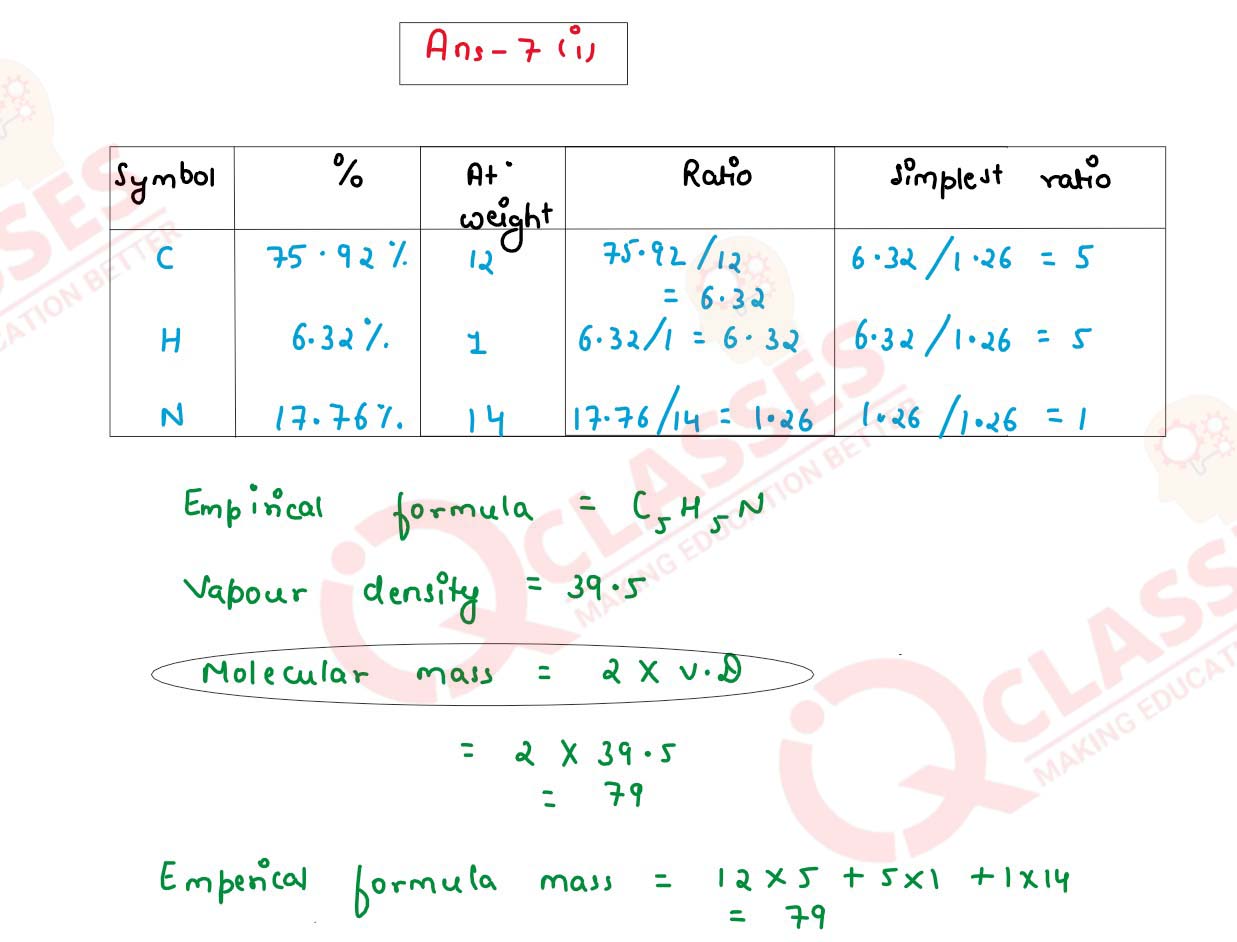
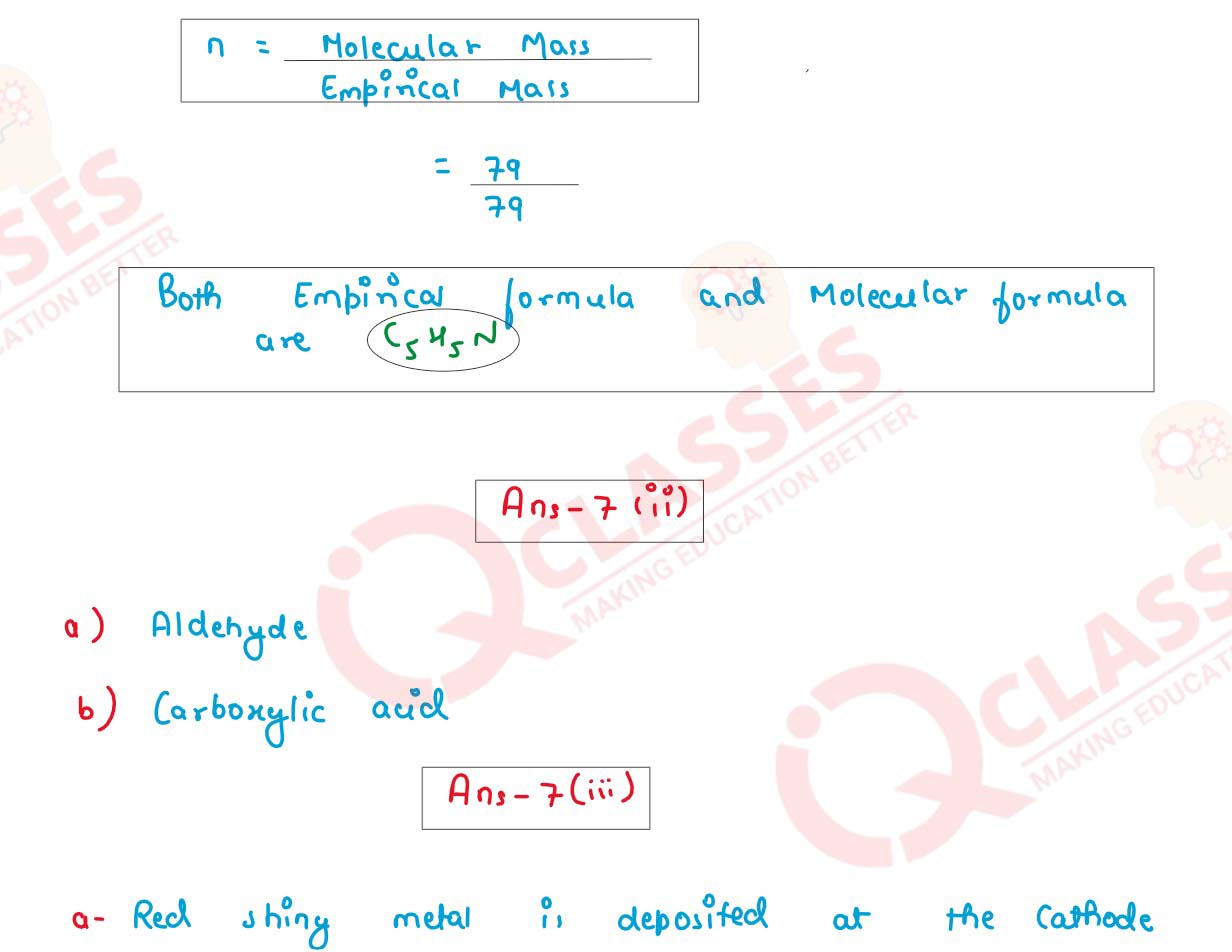
(i) Find the empirical formula and molecular formula of an organic compound from the
data given below:
C= 75.92% H = 6.32%, N = 17.76% its vapour density is 39.5
(At.wt: C=12, H=1, N=14)
(ii) Identify the functional group in the following organic compounds:
(a) HCHO
(b) C2H5COOH
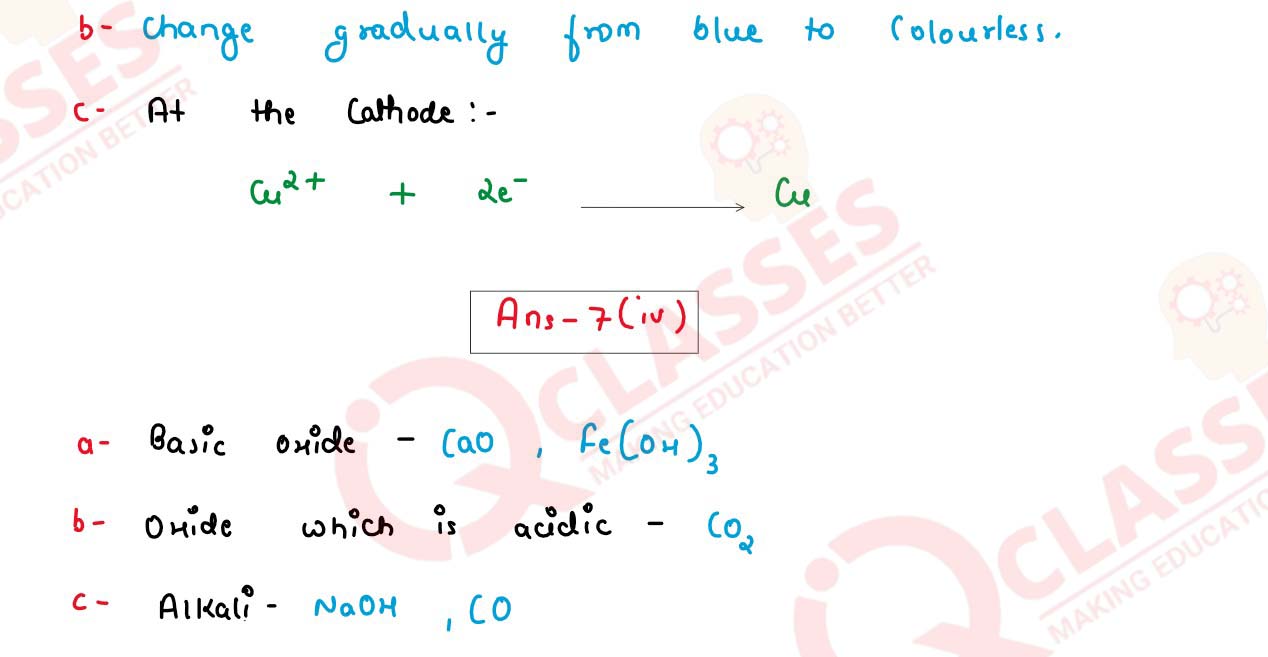
(iii) During the Electrolysis of Copper II Sulphate solution using platinum as cathode
and graphite as anode.
(a) State what you observe at the cathode.
(b) State the change noticed in the electrolyte.
(c) Write the reaction at the cathode.
(iv) Choose the answer from the list which fits the description.
[CaO, CO2, NaOH, Fe(OH)3, CO]
(a) A basic oxide.
(b) An oxide which is acidic.
(c) An Alkali.
Solution
Question 8
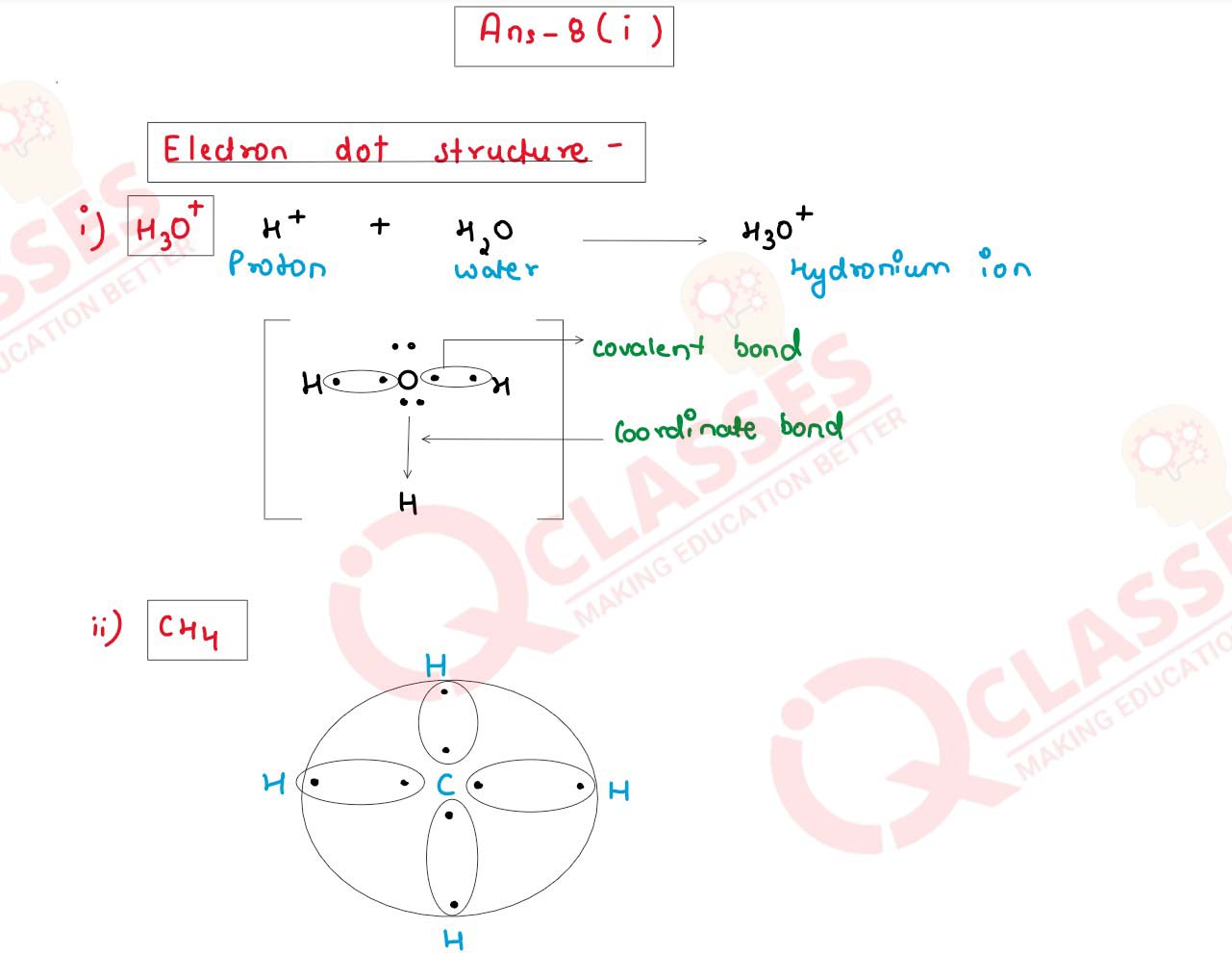
(i) Draw the electron dot structure for the following.
(a) H3O+
(b) CH4
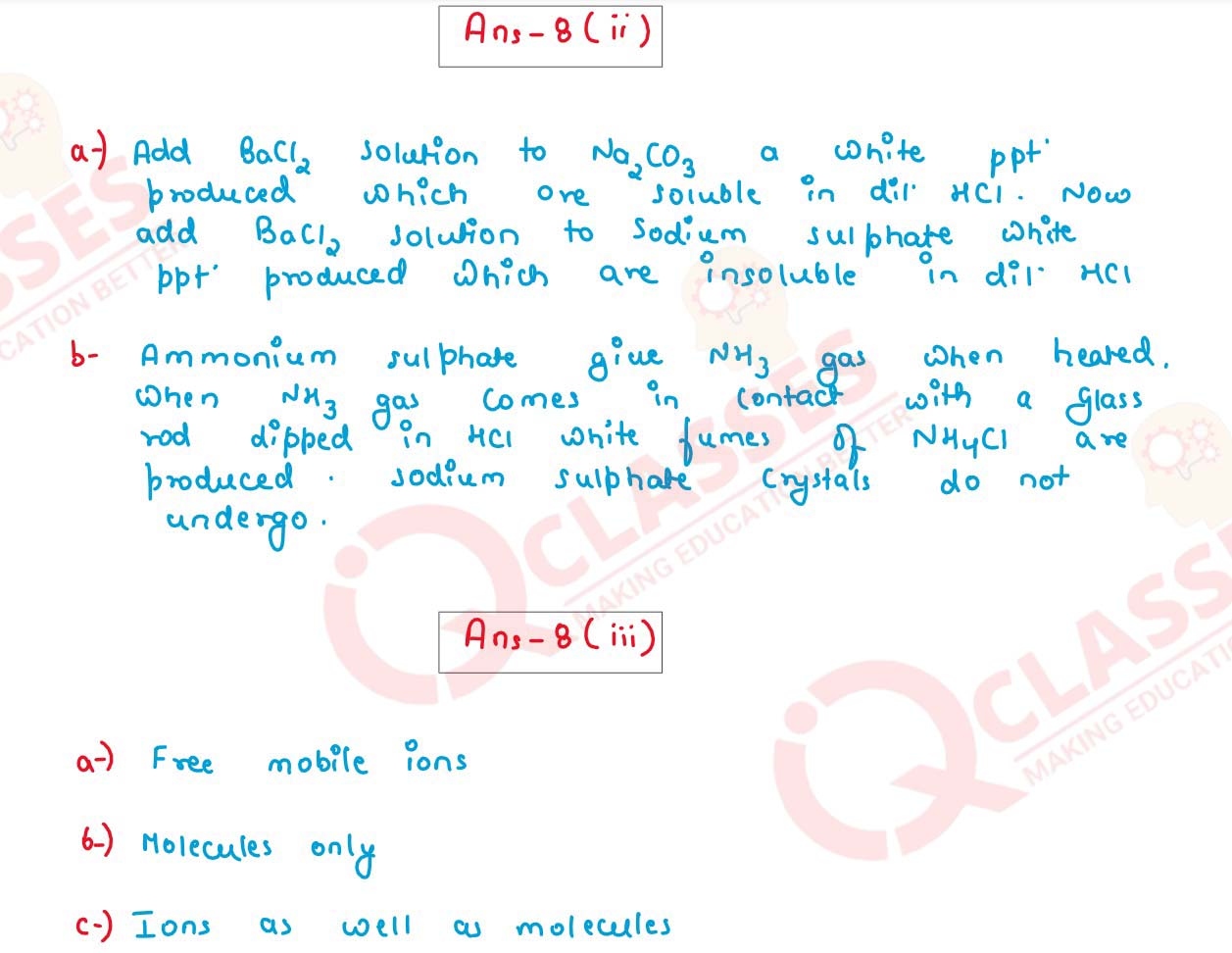
(ii) Distinguish between the following as directed:
(a) Sodium Carbonate and Sodium Sulphate by using dilute HCl
(b) Ammonium Sulphate and Sodium Sulphate by using Calcium hydroxide.
(iii) Name the particles present in:
(a) Strong Electrolyte
(b) Weak Electrolyte
(c) Non Electrolyte
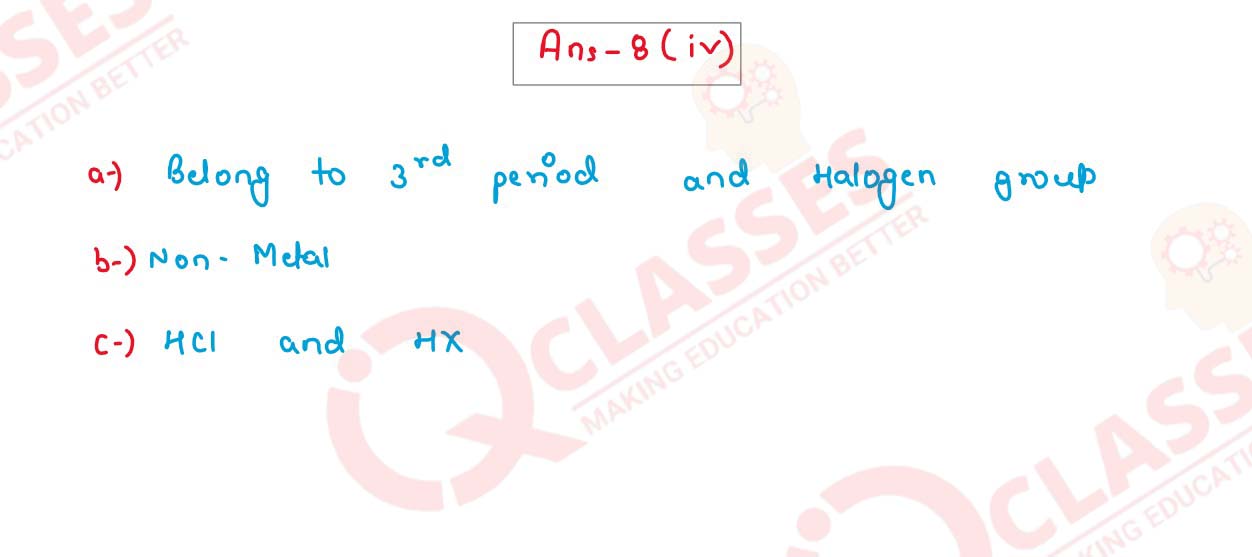
(iv) An element X has atomic number 17. Answer the following questions.
(a) State the period & group to which it belongs:
(b) Is ita Metal or Non Metal?
(c) Write the formula between X and Hydrogen.
Solution

Add a comment