Class 12 CBSE Chemistry Specimen Question 2024
Maximum Marks: 80
Time Allowed: Three hours
(i) All questions are compulsory.
(ii) The question paper has five sections and 33 questions. All questions are compulsory.
(iii) Section–A has 16 questions of 1 mark each; Section–B has 5 questions of 2 marks each; Section– C
has 7 questions of 3 marks each; Section– D has 2 case-based questions of 4 marks each; and
Section–E has 3 questions of 5 marks each.
(iv) There is no overall choice. However, internal choices have been provided in some questions. A
student has to attempt only one of the alternatives in such questions.
(v) Wherever necessary, neat and properly labeled diagrams should be drawn.
SECTION A
Question 1
Which of the following solutions will have the highest conductivity at 298 K?
(a) 0.01 M HCl solution
(b) 0.1 M HCl solution
(c) 0.01 M CH 3COOH solution
(d) 0.1 M CH 3COOH solution
Solution
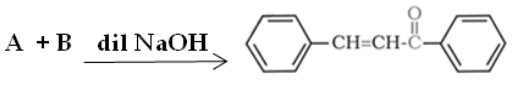
Identify A and B:
(a) A = 1-phenylethanal , B = acetophenone
(b) A = Benzophenone B = formaldehyde
(c) A= Benzaldehyde , B = Acetophenone
(d) A = Benzophenone , B = Acetophenone
Solution
The vitamins which can be stored in our body are:
(a) Vitamin A, B, D and E (d) Vitamin A, C, D and K
(c) Vitamin A, B, C and D (d) Vitamin A, D, E and K
Solution
What is IUPAC name of the ketone A, which undergoes iodoform reaction to give
CH3 CH= C(CH 3)COONa and yellow precipitate of CHI 3 ?
(a) 3-Methylpent-3-en-2one (b) 3-Methylbut-2-en- one
(c) 2, 3-Dimethylethanone (d) 3-Methylpent-4-one
Solution
Which of the following is not correct?
(a) In haloarenes, the electron pairs on halogen atom are in conjugation with π-electrons of the
ring.
(b) The carbon-magnesium bond is covalent and non-polar in nature.
(c) During SN
1 reaction, the carbocation formed in the slow step being sp2 hybridised is planar.
(d) Out of CH2= CH-Cl and C6H5CH2Cl, C6H 5CH2Cl is more reactive towards SN
1 reaction
Solution
Match the properties with the elements of 3d series:
(i) lowest enthalpy of atomisation (p) Sc
(ii) shows maximum number of oxidation states (q) Mn
(iii) transition metal that does not form coloured compounds (r) Zn
(s) Ti
(a) (i) (r), (ii) (q), (iii) (p) (b) (i) (r), (ii) (s), (iii) (p)
(c) (i) (p), (ii) (q), (iii) (r) (d) (i) (s), (ii) (r), (iii) (p)
Solution
Which of the following statement is true?
(a) molecularity of reaction can be zero or a fraction.
(b) molecularity has no meaning for complex reactions.
(c) molecularity of a reaction is an experimental quantity
(d) reactions with the molecularity three are very rare but are fast.
Solution
In which of the following solvents the C4H 8NH 3
+X-
is soluble;
(a) ether (b) acetone (c) water (d) bromine water
Solution
Which of the following observation is shown by 2 -phenyl ethanol with Lucas Reagent?
(a) Turbidity will be observed within five minutes
(b) No turbidity will be observed
(c) Turbidity will be observed immediately
(d) Turbidity will be observed at room temperature but will disappear after five minutes
Solution
If the initial concentration of substance A is 1.5 M and after 120 seconds the concentration of
substance A is 0.75 M, the rate constant for the reaction if it follows zero - order kinetics is:
(a) 0.00625 molL-1s
-1
(b) 0.00625 s
-1
(c) 0.00578 molL-1s
-1
(d) 0.00578 s
-1
Solution
Anisole undergoes bromination with bromine in ethanoic acid even in the absence of iron (III)
bromide catalyst
(a) Due to the activation of benzene ring by the methoxy group.
(b) Due to the de-activation of benzene ring by the methoxy group.
(c) Due to the increase in electron density at ortho and para positions
(d) Due to the formation of stable carbocation.
Solution
The trend of which property is represented by the following graph?
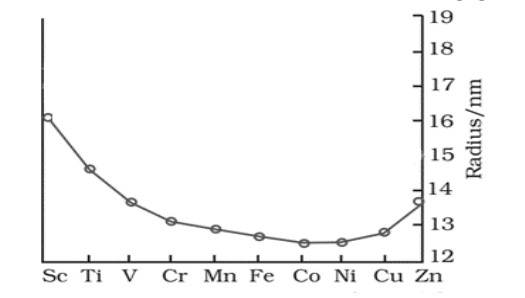
(a) ionization enthalpy (b) atomic radii
(c) enthalpy of atomization (d) melting point
Solution
Which of the following is not considered a transition element?
(a) Scandium (b) Silver (c) Vanadium (d) Zinc
Solution
r>
Given below are two statements labelled as Assertion (A) and Reason (R)
Assertion (A): Alcohols react both as nucleophiles and electrophiles.
Reason (R): The bond between C–O is broken when alcohols react as nucleophiles.
Select the most appropriate answer from the options given below:
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true
Solution
Given below are two statements labelled as Assertion (A) and Reason (R)
Assertion (A): Strong oxidising agents oxidise toluene and its derivatives to benzoic acids.
Reason (R): It is possible to stop the oxidation of toluene at the aldehyde stage with suitable
reagents.
Select the most appropriate answer from the options given below:
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Solution
Given below are two statements labelled as Assertion (A) and Reason (R)
Assertion (A): Enzymes are very specific for a particular reaction and for a particular substrate.
Reason (R): Enzymes are biocatalysts.
Select the most appropriate answer from the options given below:
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Solution
Given below are two statements labelled as Assertion (A) and Reason (R)
Assertion (A): During electrolysis of aqueous copper sulphate solution using copper electrodes
hydrogen gas is released at the cathode.
Reason (R): The electrode potential of Cu2+ /Cu is greater than that of H +/H 2
Select the most appropriate answer from the options given below:
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Solution
SECTION B
Question 17
a. Radioactive decay follows first - order kinetics. The initial amount of two radioactive
elements X and Y is 1 gm each. What will be the ratio of X and Y after two days if their halflives are
12 hours and 16 hours respectively?
b. The hypothetical reaction P + Q R is half order w.r.t ‘P’ and zero order w.r.t ‘Q’. What
is the unit of rate constant for this reaction?
Solution
A 5% solution of Na2SO4.10H 2O (MW = 3 22) is isotonic with 2% solution of non- electrolytic, non volatile substance X. Find out the molecular weight of X.
Solution
(a) Arrange the isomeric dichlorobenzene in the increasing order of their boiling point and melting
points.
(b) Explain why the electrophilic substitution reactions in haloarenes occur slowly and require
more drastic conditions as compared to those in benzene.
Solution
(a) Out of p-tolualdehyde and p-nitrobenzaldehyde, which one is more reactive towards
nucleophilic addition reactions, why?
(b) Write the structure of the product formed when acetone reacts with 2,4 DNP reagent.
OR
Convert the following:
(a) Benzene to m-nitrobenzaldehyde
(b) Bromobenzene to benzoic acid
Solution
(a) DNA fingerprinting is used to determine paternity of an individual. Which property of DNA
helps in the procedure?
(b) What structural change will occur when a native protein is subjected to change in pH?
Solution
SECTION C
Question 22
(a) Write the formula for the following coordination compound
Bis(ethane-1,2-diamine) dihydroxidochromium(III) chloride
(b) Does ionization isomer for the following compound exist? Justify your answer.
Hg[Co(SCN) 4]
(c) Is the central metal atom in coordination complexes a Lewis acid or a Lewis base? Explain.
Solution
(a) Can we construct an electrochemical cell with two half-cells composed of ZnSO4 solution and
zinc electrodes? Explain your answer.
(b) Calculate the λ0m for Cl- ion from the data given below:
Λ0m MgCl2 = 258.6 Scm2mol-1 and λ
0m Mg2+ = 106 Scm2mol-1
(c) The cell constant of a conductivity cell is 0.146 cm-1. What is the conductivity of 0.01 M solution
of an electrolyte at 298 K, if the resistance of the cell is 1000 ohm?
Solution
Write the name of the reaction, structure and IUPAC name of the product formed when (any 2):
(a) phenol reacts with CHCl3 in the presence of NaOH followed by hydrolysis.
(b) CH 3CH2CH(CH 3)CH(CH 3)ONa reacts with C2H 5Br
(c) CH 3CH2CN reacts with stannous chloride in the presence of hydrochloric acid followed by
hydrolysis
Solution
You are given four organic compounds “A”, “B”, “C” and “D”. The compounds “A”, “B” and “C”
form an orange- red precipitate with 2,4 DNP reagent. Compounds “A” and “B” reduce Tollen’s
reagent while compounds “C” and “D” do not. Both “B” and “C” give a yellow precipitate when
heated with iodine in the presence of NaOH. Compound “D” gives brisk effervescence with sodium
bicarbonate solution. Identify “A”, “B”, “C” and “D” given the number of carbon atoms in three of
these carbon compounds is three while one has two carbon atoms. Give an explanation for y our
answer.
Solution
When sucrose is hydrolysed the optical rotation values are measured using a polarimeter and are
given in the following table:
(a) Account for the two specific rotation values.
(b) What is the specific name given to sucrose based on the above observation?
(c) One of the products formed during the hydrolysis of sucrose is a glucose, that reacts with
hydroxylamine to give compound A. Identify compound A.
Solution
An organic compound A with the molecular formula (+)C 4H9Br undergoes hydrolysis to form (+) C4H9OH. Give the structure of A and write the mechanism of the reaction
Solution
The rate constants of a reaction at 200K and 500K are 0.02s-1 and 0.20s-1 respectively. Calculate the value of Ea (Given 2.303R = 19.15 JK -1mol-1)
Solution
SECTION D
Q. No. 29 and 30 are case-based questions. Each question has 3 subparts with internal choice in one subpart.
Metal complexes show different colours due to d-d transitions. The complex absorbs light of specific
wavelength to promote the electron from t2g to eg level. The colour of the complex is due to the
transmitted light, which is complementary of the colour absorbed.
The wave number of light absorbed by different complexes of Cr ion are given below:
 Answer the following questions:
Answer the following questions:
(a) Out of the ligands “A”, “B”, “C” and “D”, which ligand causes maximum crystal field splitting?
Why?
OR
Which of the two, “A” or “D” will be a weak field ligand? Why?
(b) Which of the complexes will be violet in colour? [CrA 6]
3- or [CrB6]
3+ and why? (Given: If 560 -
570 nm of light is absorbed, the colour of the complex observed is violet.)
(c) If the ligands attached to Cr3+ ion in the complexes given in the table above are water, cyanide
ion, chloride ion, and ammonia (not in this order)
Identify the ligand, write the formula and IUPAC name of the following:
(i) [CrA6]3-
(ii) [CrC6]3+
Solution
The lead-acid battery represents the oldest rechargeable battery technology. Lead acid batteries can
be found in a wide variety of applications including small-scale power storage such as UPS systems,
ignition power sources for automobiles, along with large, grid-scale power systems. The spongy
lead act as the anode and lead dioxide as the cathode. Aqueous sulphuric acid is used as an
electrolyte. The half-reactions during discharging of lead storage cells are:
Anode: Pb(s) + SO2-
(aq) → PbSO4 (s) + 2e-
Cathode: PbO (s) + 4H+
(aq) + SO2-
(aq) + 2e- → PbSO4 (s) + 2H2O
There is no safe way of disposal and these batteries end - up in landfills. Lead and sulphuric
acid are extremely hazardous and pollute soil, water as well as air. Irrespective of the
environmental challenges it poses, lead-acid batteries have remained an important source of
energy.
Designing green and sustainable battery systems as alternatives to conventional means
remains relevant. Fuel cells are seen as the future source of energy. Hydrogen is considered a
green fuel. Problem with fuel cells at present is the storage of hydrogen. Currently, ammonia
and methanol are being used as a source of hydrogen for fuel cell. These are obtained
industrially, so add to the environmental issues.
If the problem of storage of hydrogen is overcome, is it still a “green fuel?” Despite being the
most abundant element in the Universe, hydrogen does not exist on its own so needs to be
extracted from the water using electrolysis or separated from carbon fossil fuels. Both of
these processes require a significant amount of energy which is currently more than that
gained from the hydrogen itself. In addition, this extraction typically requires the use of fossil
fuels. More research is being conducted in this field to solve these problems. Despite the
problem of no good means to extract Hydrogen, it is a uniquely abundant and renewable
source of energy, perfect for our future zero-carbon needs.
Answer the following questions:
(a) How many coulombs have been transferred from anode to cathode in order to consume one
mole of sulphuric acid during the discharging of lead storage cell?
(b) How much work can be extracted by using lead storage cell if each cell delivers about
2.0 V of voltage? (1 F = 96500 C)
(c) Do you agree with the statement – “Hydrogen is a green fuel.” Give your comments for and
against this statement and justify your views.
OR
Imagine you are a member of an agency funding scientific research. Which of the following
projects will you fund and why?
(i) safe recycling of lead batteries
(ii) extraction of hydrogen
Solution
SECTION E
Question 31
Attempt any five of the following:
(a) Which of the following ions will have a magnetic moment value of 1.73 BM.
Sc3+, Ti3+, Ti2+, Cu2+, Zn2+
(b) In order to protect iron from corrosion, which one will you prefer as a sacrificial electrode, Ni or
Zn? Why? (Given standard electrode potentials of Ni, Fe and Zn are -0.25 V, -0.44 V and -0.76 V
respectively.)
(c) The second ionization enthalpies of chromium and manganese are 1592 and 1509 kJ/mol
respectively. Explain the lower value of Mn.
(d) Give two similarities in the properties of Sc and Zn.
(e) What is actinoid contraction? What causes actinoid contraction?
(f) What is the oxidation state of chromium in chromate ion and dichromate ion?
(g) Write the ionic equation for reaction of KI with acidified KMnO 4.
Solution
(a) What is the effect of temperature on the solubility of glucose in water?
(b) Ibrahim collected a 10mL each of fresh water and ocean water. He observed that one sample
labeled “P” froze at 0 oC while the other “Q” at -1.3oC. Ibrahim forgot which of the two, “P” or
“Q” was ocean water. Help him identify which container contains ocean water, giving
rationalization for your answer.
(c) Calculate Van't Hoff factor for an aqueous solution of K3 [Fe(CN)6] if the degree of dissociation
(α) is 0.852. What will be boiling point of this solution if its concentration is 1 molal? (Kb=0.52 K
kg/mol)
OR
(a) What type of deviation from Roult’s Law is expected when phenol and aniline are mixed with
each other? What change in the net volume of the mixture is expected? Graphically represent
the deviation.
(b) The vapour pressure of pure water at a certain temperature is 23.80 mm Hg. If 1 mole of a
nonvolatile non- electrolytic solute is dissolved in 100g water, Calculate the resultant vapour
pressure of the solution.
Solution
An organic compound with molecular formula C7H7NO2 exists in three isomeric forms, the
isomer ‘A’ has the highest melting point of the three. ‘A’ on reduction gives compound ‘B’ with
molecular formula C7H9N. ‘B’ on treatment with NaNO2/HCl at 0-5 0C to form compound ‘C’.
On treating C with H3PO2 ,it gets converted to D with formula C7H8 , which on further reaction
with CrO2Cl2 followed by hydrolysis forms ‘E’ C7H6O . Write the structure of compounds A to
E . Write the chemical equations involved.
(a) Account for the following:
(i) N-ethylbenzenesulphonyl amide is soluble in alkali .
(ii) Reduction of nitrobenzene using Fe and HCl is preferred over Sn and HCl.
(b) Arrange the following in:
(i) decreasing order of pKb values
C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3
(ii) increasing order of solubility in water
C2H5Cl, C2H5NH2, C2H5OH
(iii) decreasing boiling point
CH3COOH, C2H5OH, CH3NH2, CH3OCH3
Solution

Add a comment