CLASS 12 CBSE TERM1 CHEMISTRY SPECIMEN 2022
BOARD -
CLASS -
SUBJECT -
CBSE
12th
CHEMISTRY
Paper Pattern for MCQ Term-I
TIME -
MARKS -
1 Hour 30 Minutes
35
Visit CBSE OFFICIAL PAGE for Regulations and Syllabus of Class 12th CBSE
Solved Specimen Paper Semester-I 2022
SECTION- A
Q.1 Which of the following statements is true:
- Melting point of Phosphorous is less than that of Nitrogen
- N2 is highly reactive while P4 is inert
- Nitrogen shows higher tendency of catenation than P
- N-N is weaker than P-P
Solution

Q.2 Which of the following is a non-stoichiometric defect?
- Frenkel defect
- Schottky defect
- metal deficiency defect
- interstitial defect
Solution

Q.3 Identify the law which is stated as: “For any solution, the partial vapour pressure of each volatile component in the solution is directly proportional to its mole fraction.”
- Henry’s law
- Raoult’s law
- Dalton’s law
- Gay-Lussac's Law
Solution

Q.4 Pink colour of LiCl crystals is due to:
- Schottky defect
- Frenkel defect
- Metal excess defect
- Metal deficiency defect
Solution

Q.5 Which of the following isomer has the highest meltingpoint:
- 1,2-dicholorbenzene
- 1,3 -dichlorobenzene
- 1,4-dicholorbenzene
- all isomers have same melting points
Solution

Q.6 Which one of the following reactions is not explained by the open chain Structureof glucose:
- Formation of pentaacetate of glucose with acetic anhydride.
- formation of addition product with 2,4 DNP reagent
- Silver mirror formation with Tollen’s reagent
- existence of alpha and beta forms of glucose
Solution
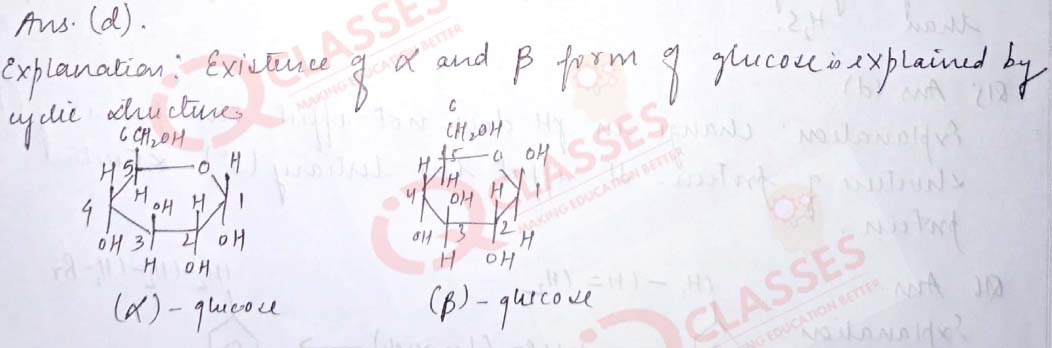
Q.7 Williamson’s synthesis of preparing dimethyl ether is an:
- SN1reaction
- Elimination reaction
- SN2reaction
- Nucleophilic addition reaction
Solution
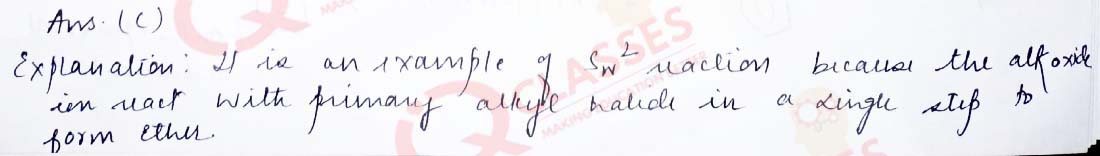
Q.8 Chlorine water loses its yellow colour on standing because:
- HCl gas is produced, due to the action of sunlight
- a mixture of HOCl and HCl is produced in the presence of light
- HOCl and hydrogen gas is produced
- a mixture of HCl and ClO3 is produced, due to the action of sunlight
Solution

Q.9 During dehydration of alcohols to alkenes by heating with concentrated H2SO4, the initiation step is:

- protonation of alcohol molecule
- formation of carbocation
- elimination of water
- formation of an ester
Solution

Q.10 Amorphous solids are:
- isotropic
- anisotropic
- isotopic
- isomeric
Solution

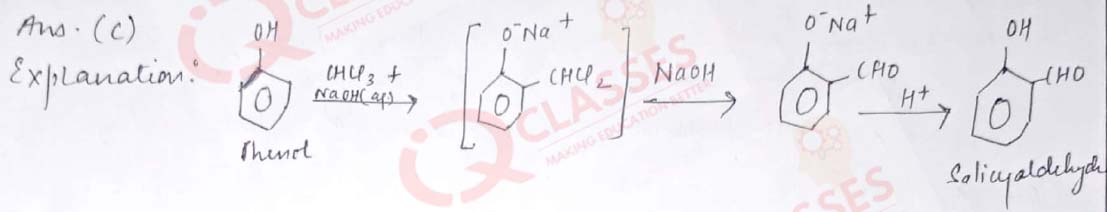
Q.11 Which of the following reactions is used to prepare salicylaldehyde?

- Kolbe’s reaction
- Etard reaction
- Reimer- Tiemann reaction
- Stephen’s reduction.
Solution
Q.12 Which of the following is an example of a solid solution?
- sea water
- sugar solution
- smoke
- 22 carat gold
Solution

Q.13 The boiling points of alcohols are higher than those of hydrocarbons of comparable masses due to:
- Hydrogen bonding
- Ion – dipole interaction
- Dipole- dipole interaction
- Van der Waal’s forces
Solution
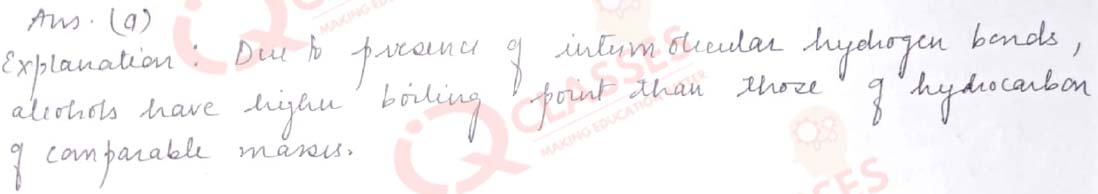
Q.14 Which of the following has the lowest boiling point:
- H2O
- H2S
- H2Se
- H2Te
Solution
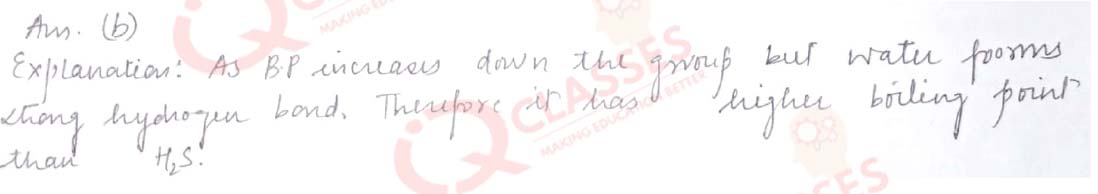
Q.15 Which of the following statement is correct:
- Fibrous proteins are generally soluble in water
- Albumin is an example of fibrous proteins
- In fibrous proteins, the structure is stabilised by hydrogen bonds and disulphide bonds
- pH does not affect the primary structure of protein
Solution

Q.16 Major product obtained on reaction of 3-Phenyl propene with HBr in presence of organic peroxide
- 3- Phenyl 1- bromopropane
- 1 –Phenyl -3- bromopropane
- 1-Phenyl -2-bromopropane
- 3-Phenyl -2- bromopropane
Solution
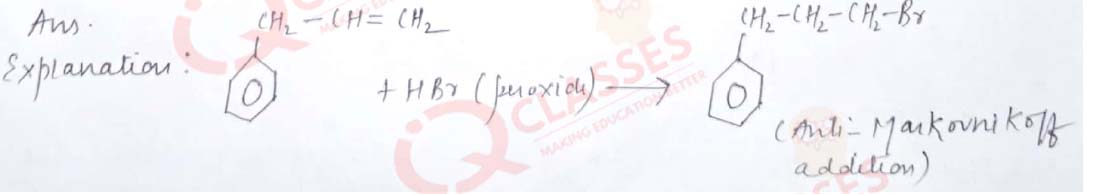
Q.17 Which of the following is a correct statement for C2H5Br?
- It reacts with metallic Na to give ethane
- It gives nitroethane on heating with aqueous solution of AgNO2
- It gives C2H5OH on boiling with alcoholic potash
- It forms diethylthioether on heating with alcoholic KSH.
Solution

Q.18 Covalency of nitrogen is restricted to:
- 2
- 3
- 4
- 5
Solution
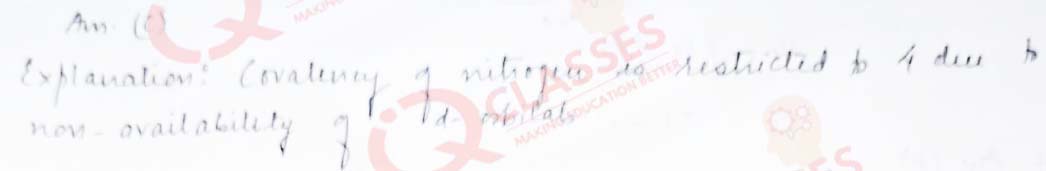
Q.19 Solubility of gases in liquids decreases with rise in temperature because dissolution is an:
- endothermic and reversible process
- exothermic and reversible process
- endothermic and irreversible process
- exothermic and irreversible process
Solution
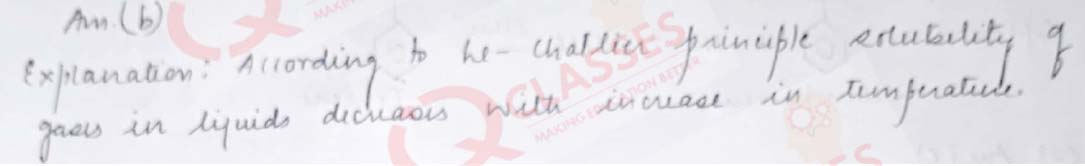
Q.20 All elements of Group 15 show allotropy except:
- Nitrogen
- Arsenic
- Antimony
- Bismuth
Solution
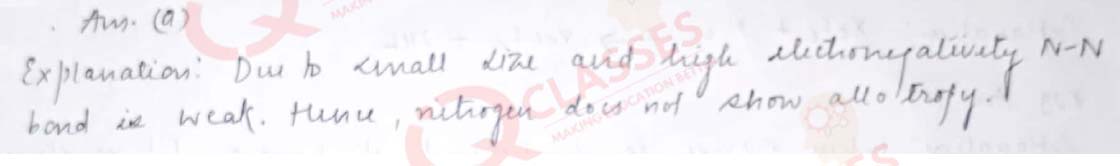
Q.21 Which of the following is a polysaccharide?
- glucose
- maltose
- glycogen
- lactose
Solution

Q.22 Substance having the lowest boiling point:
- Hydrogen
- Oxygen
- Nitrogen
- Helium
Solution

Q.23 Lower molecular mass alcohols are:
- miscible in limited amount of water
- miscible in excess of water
- miscible in water in all proportions
- immiscible in water
Solution

Q.24 Maximum oxidation state exhibited by Chlorine is:
- +1
- +3
- +5
- +7
Solution
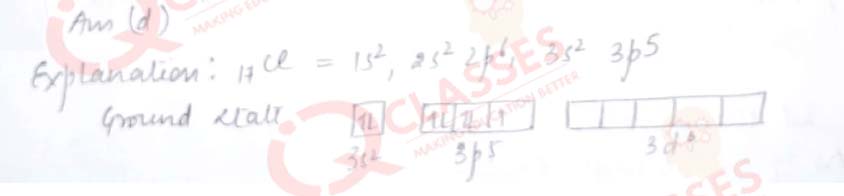
Q.25 In which of the following cases blood cells will shrink:
- when placed in water containing more than 0.9% (mass/ volume) NaCl solution.
- when placed in water containing less than 0.9% (mass /volume) NaCl solution.
- when placed in water containing 0.9% (mass/volume) NaCl solution
- when placed in distilled water.
Solution

Q.26 How much ethyl alcohol must be added to 1 litre of water so that the solution will freeze at– 14°C ? (Kf for water = 1.86°C/mol)
- 7.5 mol
- 8.5 mol
- 9.5 mol
- 10.5 mol
Solution
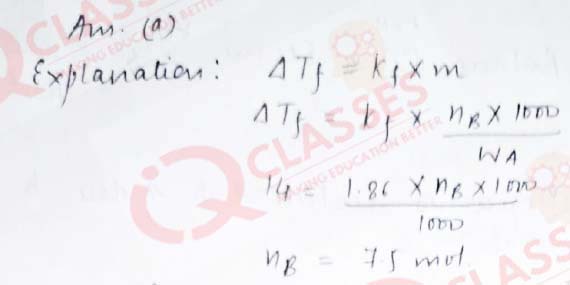
Q.27 Which reagents are required for one step conversion of chlorobenzene to toluene?
- CH3Cl / AlCl3
- CH3Cl, Na, Dry ether
- CH3Cl/Fe dark
- NaNO2/ HCl /0-5oC
Solution
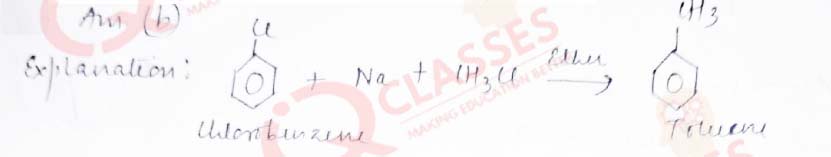
Q.28 On partial hydrolysis, XeF6 gives:
- XeO3 +4HF
- XeO2F + HF
- XeOF4+ H2
- XeO2F2 + 4HF
Solution

Q.29 Which one of the following statement is correct about sucrose :
- It can reduce tollen’s reagent however cannot reduce fehling’s reagent
- It undergoes mutarotation like glucose and fructose
- It undergoes inversion in the configuration on hydrolysis
- It is laevorotatory in nature
Solution

Q30. Phenol does not undergo nucleophilic substitution reaction easily due to:
- acidic nature of phenol
- partial double bond character of C-OH bond
- partial double bond character of C-C bond
- instability of phenoxide ion
Solution

Q.31 Which of the following has highest ionisation enthalpy?
- Nitrogen
- Phosphorus
- Oxygen
- Sulphur
Solution

Q.32 Metal M ions form accp structure. Oxide ions occupy ½ octahedral and ½ tetrahedral voids. What is the formula of the oxide?
- MO
- MO2
- MO3
- M2O3
Solution

Q.33 The reaction of toluene with Cl2 in presence of FeCl3 gives ‘X’ while theof toluene with Cl2 in presence of light gives ‘Y’. Thus ‘X’ and ‘Y’ are:
- X = benzyl chloride Y = o and p – chlorotoluene
- X = m – chlorotoluene Y = p – chlorotoluene
- X = o and p–chlorotoluene Y = trichloromethylbenzene
- X= benzyl chloride, Y = m-chlorotoluene
Solution

Q.34 Ozone is a/ an __________ molecule and the two O-O bond lengths in ozone are (i)_______-and (ii) ____________
- linear ,110pm ; 148pm
- angular, 110pm ; 148pm
- linear, 128pm ; 128pm
- angular, 128pm ; 128pm
Solution

Q.35 Water retention or puffiness due to high salt intake occurs due to:
- diffusion
- vapour pressure difference
- osmosis
- reverse osmosis
Solution
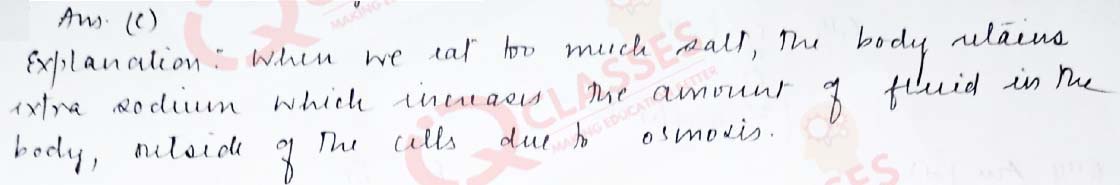
Q.36 In the following reaction,identify A and B:

- A= COOH-(CH2)4 -COOH, B= OHC-(CHOCOCH3)4 -CH2OCOCH3
- A= COOH-(CH2)4 -CHO , B= OHC-(CHOCOCH3)4 -CH2OCOCH3
- A= OHC-(CHOCOCH3)3-CH2OCOCH3 B= COOH-(CH2)4 -CHO
- A= OHC-(CHOCOCH3)4-CH2OCOCH3 B= COOH-(CH2)4 -COOH
Solution
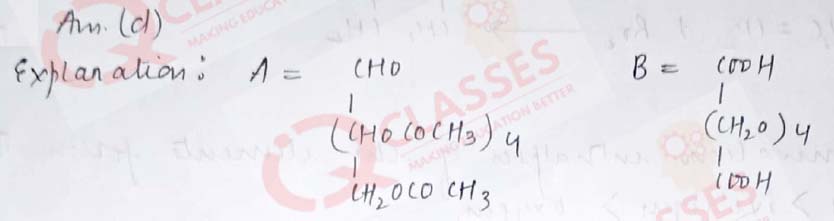
Q.37 In lake test for Al3+ ions, there is the formation of coloured ‘floating lake’. It is due to:
- Absorption of litmus by [Al(OH)4]-
- Absorption of litmus by Al(OH)3
- Adsorption of litmus by [Al(OH)4]-
- Adsorption of litmus by Al(OH)3
Solution

Q.38 A unit cell of NaCl has 4 formula units. Its edge length is 0.50 nm. Calculate the density if molar mass of NaCl = 58.5 g/mol.
- 1 g/cm3
- 2 g/cm3
- 3 g/cm3
- 4g/cm3
Solution
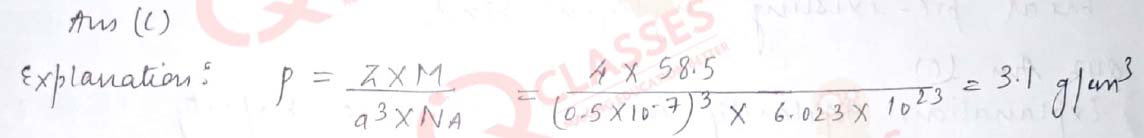
Q.39 Which one of the following are correctly arranged on the basis of the property indicated:
- I2 < Br2 < F2 < Cl2 [ increasing bond dissociation enthalpy]
- H2O > H2S < H2Te < H2Se [ increasing acidic strength]
- NH3 < N2O< NH2OH < N2O5 [ increasing oxidation state]
- BiH3 < SbH3 < AsH3 < PH3 < NH3 [ increasing bondangle]
Solution
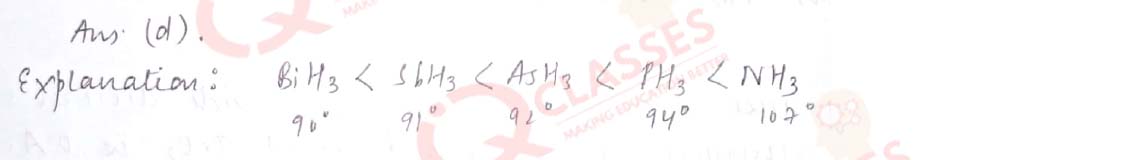
Q.40 What would be the reactant and reagent used to obtain 2, 4-dimethyl pentan-3-ol?
- Propanal and propyl magnesium bromid
- 3-methylbutanal and 2-methyl magnesium iodide
- 2-dimethylpropanone and methyl magnesium iodide
- 2- methylpropanal and isopropyl magnesium iodide
Solution
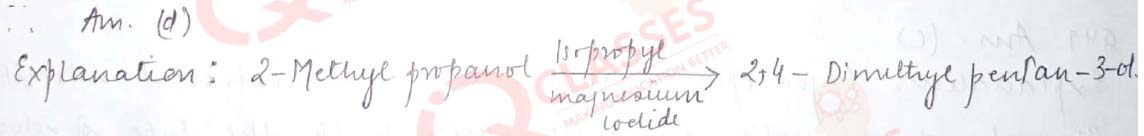
Q.41 o-hydroxy benzyl alcohol when reacted with PCl3 gives the product as (IUPAC name)
- o- hydroxy benzyl chloride
- 2- chloromethylphenol
- o-chloromethylchlorobenzene
- 4-hydroxymethylphenol
Solution

Q.42 Which of the following statements is true:
- Ammonia is the weakest reducing agent and the strongest base among Group 15 hydrides
- Ammonia is the strongest reducing agent as well as the hydrides
- Ammonia is the weakest reducing agent as well as the weakest base among Group 15 hydrides
- Ammonia is the strongest reducing agent and the weakest base among Group 15 hydrides
Solution

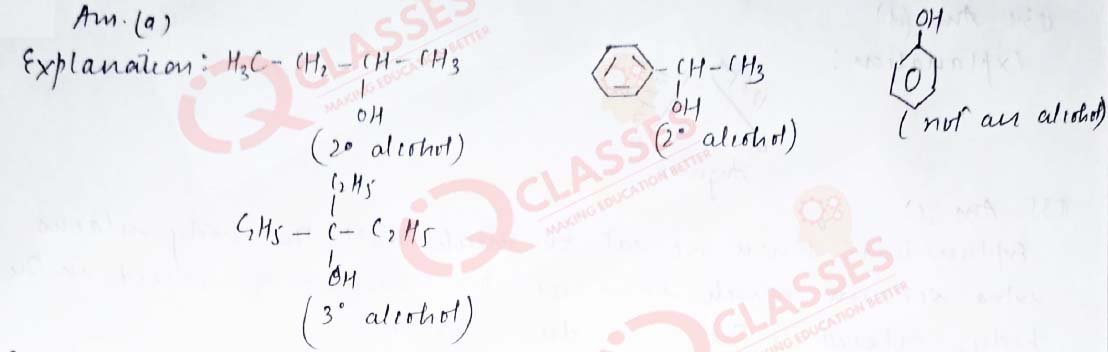
Q.43 Identify the secondary alcohols from the following set
CH3CH2CH(OH)CH3 (C2H5)3COH

- (i) and (iv)
- (i) and (iii)
- (i) and (ii)
- (i), (iii) and (iv)
Solution
Q.44 Alkenes decolourise bromine water in presence of CCl4 due to formation of:
- allyl bromide
- vinyl bromide
- bromoform
- vicinal dibromide
Solution

Q.45 Given below are two statements labelled as Assertion (A) and Reason (R) Assertion (A): Electron gain enthalpy of oxygen is less than that of Flourine but greater than Nitrogen. Reason (R): Ionisation enthalpies of the elements follow the order Nitrogen > Oxygen > Fluorine Select the most appropriate answer from the options given below:
- Both A and R are true and R is the correct explanation of A
- Both A and R are true but R is not the correct explanation of A
- A is true but R is false
- A is false but R is true
Solution
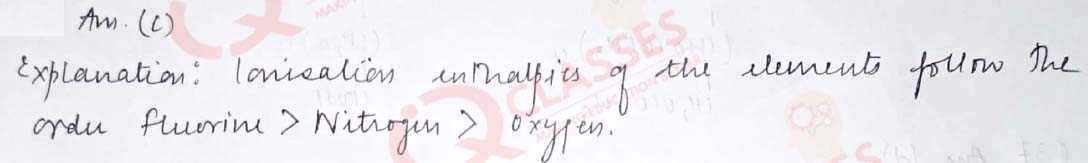
Q.46 Given below are two statements labelled as Assertion (A) and Reason (R) Assertion (A): Alkyl halides are insoluble in water. Reason (R): Alkyl halides have halogen attached to sp3 hybrid carbon. Select the most appropriate answer from the options given below:
- Both A and R are true and R is the correct explanation of A
- Both A and R are true but R is not the correct explanation of A
- A is true but R is false
- A is false but R is true
Solution

Q.47 Given below are two statements labelled as Assertion (A) and Reason (R) Assertion(A): Molarity of a solution changes with temperature. Reason (R): Molarity is a colligative property. Select the most appropriate answer from the options given below:
- Both A and R are true and R is the correct explanation of A
- Both A and R are true but R is not the correct explanation of A
- A is true but R is false
- A is false but R is true
Solution

Q.48 Given below are two statements labelled as Assertion (A) and Reason (R) Assertion(A):SO2 is reducing while TeO2 is an oxidising agent. Reason(R):Reducing property of dioxide decreases from SO2 to TeO2 . Select the most appropriate answer from the options given below:
- Both A and R are true and R is the correct explanation of A
- Both A and R are true but R is not the correct explanation of A
- A is true but R is false
- A is false but R is true
Solution
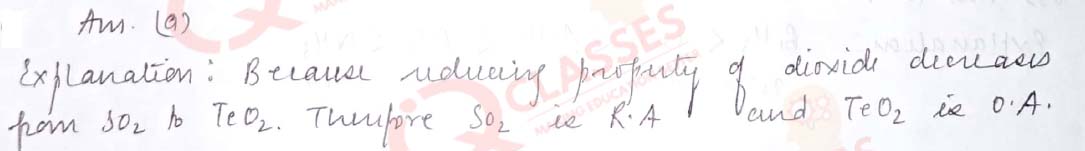
Q.49 Given below are two statements labelled as Assertion (A) and Reason (R) Assertion (A):Cryoscopic constant depends on nature of solvent. Reason(R ):Cryoscopic constant is a universal constant. Select the most appropriate answer from the options given below:
- Both A and R are true and R is the correct explanation of A
- Both A and R are true but R is not the correct explanation of A
- A is true but R is false
- A is false but R is true
Solution

Q.50
Match the following:
 Which of the following is the
best matched options?
Which of the following is the
best matched options?
- i-A, v- D, iii- C, iv-B
- i-D, ii-C, iii- A, iv-B
- i-D, v- D, iii- A, iv-B
- i-A, ii- C, iii- D, iv-B
Solution

Q.51Which of the following analogies is correct:
- Nitrogen: 1s22s22p3 :: Argon:1s22s22p6
- Carbon: maximum compounds :: Xenon: no compounds
- XeF2: Linear :: ClF3: Trigonal planar
- Helium: meteorological observations:: Argon: metallurgical processes
Solution

Q.52Complete the following analogy: Same molecular formula but different structures: A:: Non superimposable mirror images: B
- A:Isomers B: Enantiomer
- A: Enantiomers B: Racemic mixture
- A: Sterioisomers B: Retention
- A: Isomers B: Sterioisomers
Solution

CASE1: Read the passage given below and answer the following
questions 53-55Early crystallographers had trouble solving the structures of inorganic solids
using X-ray
diffraction because some of the mathematical tools for analyzing the data had not yet been developed.
Once a trial structure was proposed, it was relatively easy to calculate the diffraction pattern, but it
was difficult to go the other way (from the diffraction pattern to the
structure) if nothing was known a priori about the arrangement of atoms in the unit cell. It was
important to develop some guidelines for guessing the coordination numbers and bonding geometries of
atoms in crystals. The first such rules were proposed by Linus Pauling, who considered how one might
pack together oppositely charged spheres of different radii. Pauling proposed from geometric
considerations that the quality of the "fit" depended on the radius ratio of the anion and the cation.
If the anion is considered as the packing atom in the crystal, then the smaller cation fills
interstitial sites ("holes"). Cations will find arrangements in which they can contact the
largest number of anions. If the cation can touch all of its nearest neighbour anions then the fit is
good. If the cation is too small for a given site, that coordination number will be
unstable and it will prefer a lower coordination structure. The table below gives the ranges of
cation/anion radius ratios that give the best fit for a given coordination geometry.

Q.53The radius of Ag+ ion is 126pm and of I- ion is 216pm. The coordination number of Ag+ ion is:
- 2
- 3
- 6
- 8
Solution

Q.54 A solid AB has square planar structure. If the radius of cation A+ is 120pm, calculate the maximum possible value of anion B-
- 240 pm
- 270 pm
- 280 pm
- 290 pm
Solution

Q.55A “good fit” is considered to be one where the cation can touch:
- all of its nearest neighbour anions
- most of its nearest neighbour anions
- some of its nearest neighbour anions
- none of its nearest neighbour anions.
Solution
