Class 12 ISC term2 Chemistry Specimen 2022
BOARD -
CLASS -
SUBJECT -
ISC
12th
CHEMISTRY
Paper Pattern for MCQ Term-I
TIME -
MARKS -
1 Hour 30 Minutes
35
Visit CISCE OFFICIAL PAGE for Regulations and Syllabus of Class 12th ISC
Solved Specimen Paper Semester-2 2022
Section-A [Chemistry Specimen Paper Semester-2 2022]
Question 1
Fill in the blanks by choosing the appropriate word(s) from those given in the brackets
(two, four, sec-1 , diamagnetic, acetaldehyde, mol-1L sec-1 , paramagnetic, formaldehyde, acetone, ethanol)
(i) When the concentration of a reactant of first order reaction is doubled, the rate of reaction becomes ___________ times. The unit of rate constant (k) for the first order reaction is __________.
Solution
Two and Four
(ii) The transition metals show ___________ character because of the presence of unpaired electrons while Cu+ is ___________ because its electronic configuration is [Ar]3d10 .
Solution
paramagnetic and diamagnetic
(iii) Calcium formate on distillation gives ___________ but the distillation of calcium formate and calcium acetate gives ___________.
Solution
Acetone and formaldehyde
Question 2
Select and write the correct alternative from the choices given below
(i) The type of hybridization involved in Octahedral complexes is:
- sp3
- dsp2
- sp3d
- d2sp3
Solution
(d)
d2sp3
(ii) One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44 amu. The alkene is:
- ethene
- propene
- 1-butene
- 2-butene
Solution
(d)
2-butene
(iii) Primary amine when warmed with chloroform and alc. KOH yields:
- cyanides
- isocyanides
- benzene diazonium chloride
- secondary amines
Solution
(b)
isocyanides
(iv)
Assertion: The conversion of fresh precipitate to colloidal state is called
peptization.
Reason: It is caused by addition of common ions.
- Both assertion and reason are true and reason is the correct explanation of assertion
- Both assertion and reason are true but reason is not the correct explanation for assertion.
- Assertion is true but reason is false
- Assertion is false but reason is true.
Solution
(b)
Both assertion and reason are true but reason is not the correct explanation for assertion.
Section-B
Question 3
Name the type of isomerism shown by each of the following pairs of compounds:
(i) [CoCl2(NH3)4]Cl.H2O and [CoCl(H2O)(NH3)4]Cl2
Solution
Hydrate and Solvent isomerisn
(ii) [Cr(NH3)5Br]SO4 and [Cr(NH3)5SO4]Br
Solution
Ionisation isomerisn
Question 4
(i) Write chemical equations to illustrate each of the following name reactions:
- Rosenmund’s reduction
- Clemmensen’s reduction
Solution
.jpg)
Solution
.jpg)
OR
(ii) How will you bring about the following conversions? (Give equation).
- Acetic acid to acetone
- Formaldehyde to urotropine
Solution
.jpg)
Solution
.jpg)
Question 5
What is a zwitter ion? Represent the zwitter ion of glycine.
Solution

Question 6
- Arrange the following in the increasing order of their basic strength:
C2H5NH2,
C6H5NH2, (C2H5)2NH.
Solution
.jpg)
- What are the products formed when benzene diazonium chloride reacts with
phenol in weak alkaline medium? (Give equation).
Solution
.jpg)
Solution
.jpg)
Solution
.jpg)
Question 7
Give reasons for the following:
- Diabetic patients are advised to take artificial sweeteners instead of natural
sweeteners.
Solution
.jpg)
- The use of aspartame is limited to cold foods and drinks.
Solution
.jpg)
Solution
.jpg)
Solution
.jpg)
Question 8
The rate of reaction becomes four times when the temperature changes from 293K to
313K. Calculate the energy of activation (Ea) of the reaction assuming that it does not
change with temperature. (R = 8·314 JK-1mol-1
)
Solution
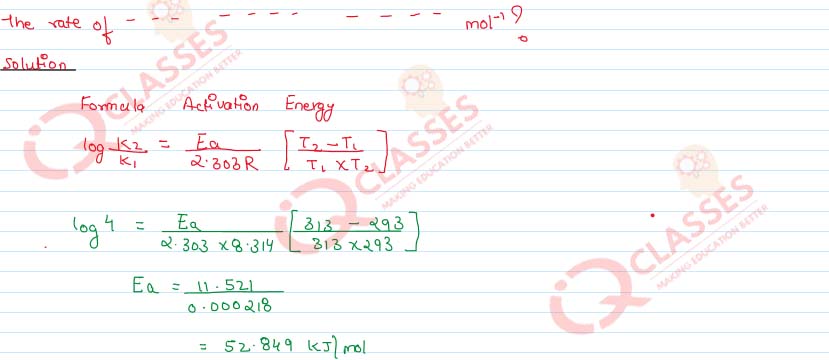
Question 9
Give balanced equation for each of the following:
(i)
Ethylamine and nitrous acid
Solution
.jpg)
(i)
Aniline and acetyl chloride
Solution
.jpg)
Question 10
Give one chemical test for each to distinguish between the following pairs of compound:
(i)
Acetaldehyde and benzaldehyde
Solution
.jpg)
(ii)
Acetone and acetic acid
Solution
.jpg)
Section-C
Question 1
Answer the following:
(a)
Define molecularity of a reaction. Give one difference between the order of
reaction and its molecularity
Solution
.jpg)
(b)
The rate constant (k) of a first order reaction is 4·5 x 10-2
sec-1
. What will be
the time required for the initial concentration of 0·4 M of the reactant to be
reduced to 0·2 M?
Solution
.jpg)
OR
Answer the following:
(a)
For a first order reaction, show that the time required for the completion of
99% reaction is twice the time required for the completion of 90% of the
reaction.
Solution
.jpg)
(b)
For a reaction, rate = k[A]1
[B]1.5[C]0
. What is the overall order of reaction?
Solution
.jpg)
Question 12
(i)
What is the basic difference between the electronic configuration of transition
and inner transition elements?
Solution
.jpg)
(ii)
Why are Zn2+ ions colourless while Ni2+ ions are green in colour?
Solution
.jpg)
Question 13
(i) Write the formula of each of the following compounds:
- Potassium trioxalatoaluminate (III)
- Triammine triaquachromium (III) chloride
Solution
.jpg)
(ii) For the complex ion [Co(NH3)6]3+ , state the oxidation state of central metal atom and the coordination number of the complex ion.
Solution
.jpg)
Question 14
(i) For ferric hydroxide sol. the coagulating power of phosphate ion is more than chloride ion
Solution
.jpg)
(ii) Lyophilic colloidal specimenchapimg/isc-chem-speci are more stable than lyophobic colloidal specimenchapimg/isc-chem-speci.
Solution
.jpg)
(iii) Gelatin is added to ice cream.
Solution
.jpg)