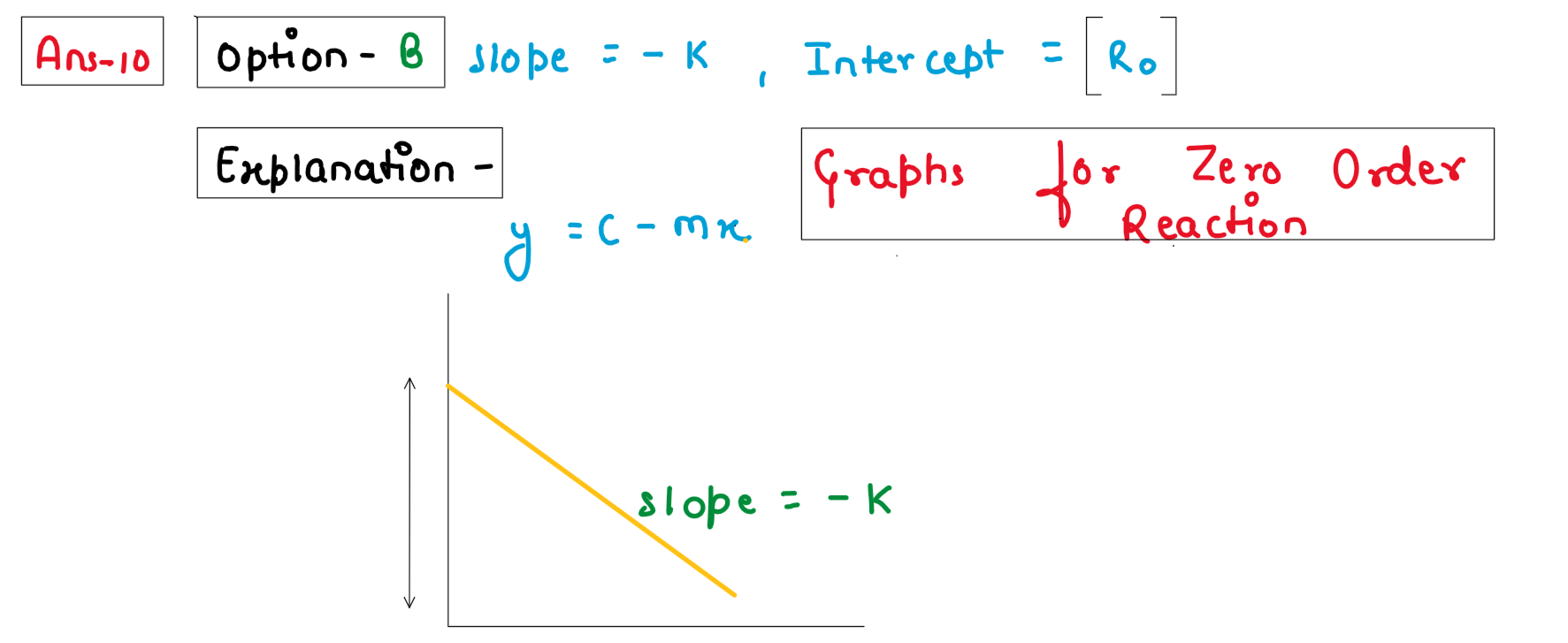
In a given graph of zero order reaction, the slope and intercept are :
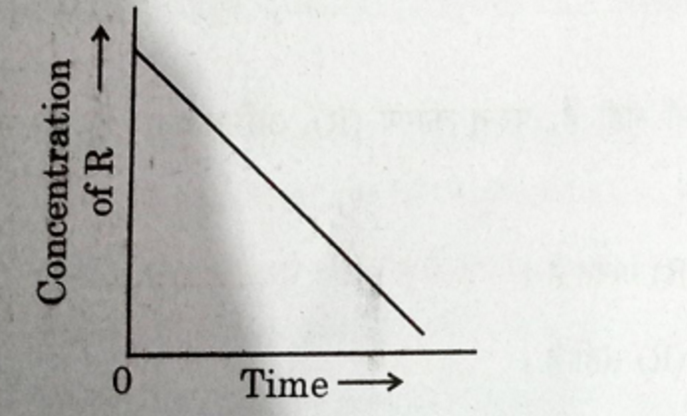
(A) Slope = k, Intercept = [R]o
(B) Slope = -k, Intercept = [R]o
(C) Slope = k/2.303, Intercept =In[R]o
(D) Slope = -k/2.303, Intercept = In A
Solution