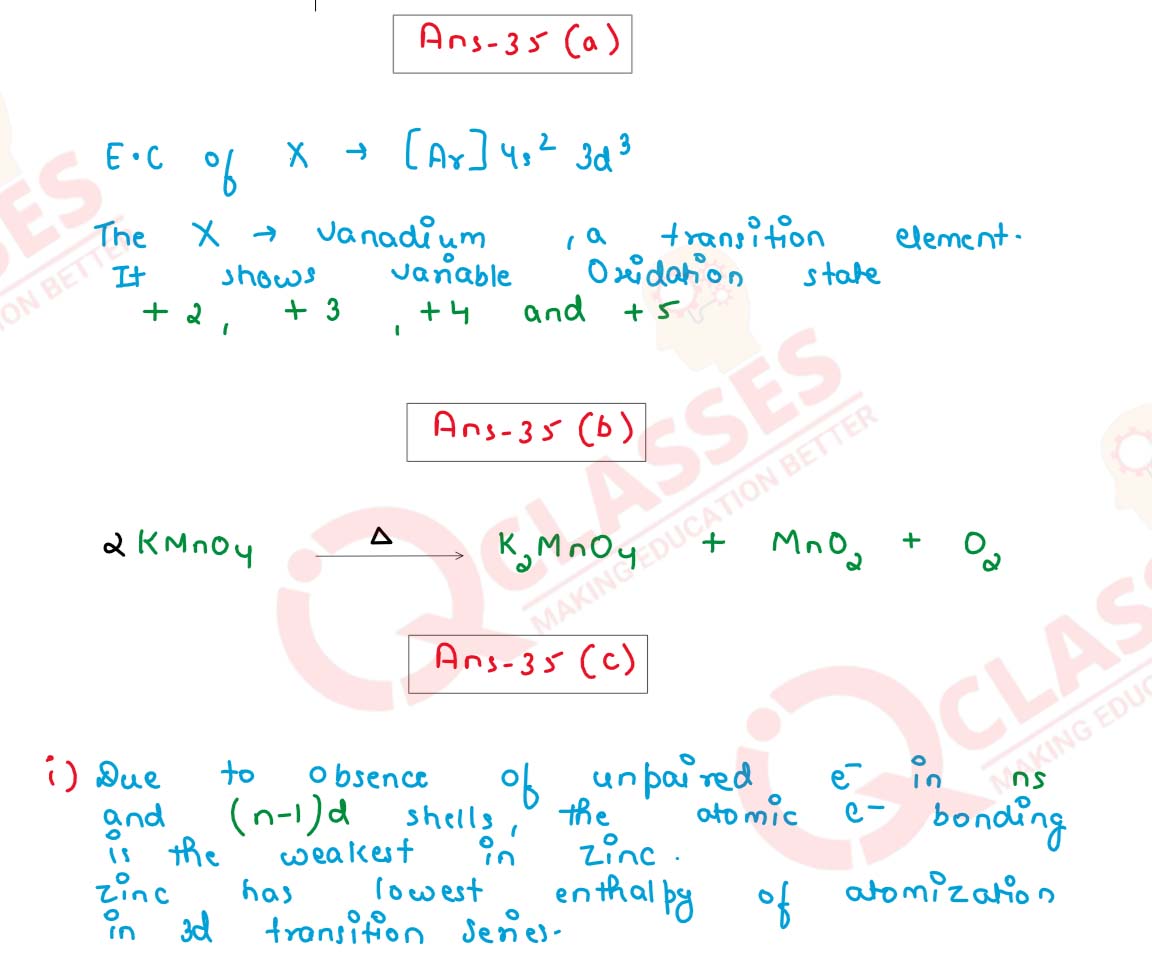
(a) A transition element X has electronic configuration [Ar] 4s2 3d3. Predict its likely oxidation states.
(b) Complete the reaction mentioning all the products formed :
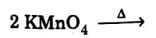
(c) Account for the following :
(i) In the 3d transition series, zinc has the lowest enthalpy of atomisation.
(ii) Cu+ ion 1s unstable in aqueous solution.
(iii) Actinoids show more number of oxidation states than lanthanoids.
Solution
 ,
,