(a) (i) Define reverse osmosis
(ii) Why are aquatic species more comfortable in cold in comparison to warm water?
(iii) A solution containing 2g of glucose (M=180 gmol-1) in 100g of water is prepared at 303K.If the vapour pressure of pure water at 303K is 32.8 mm Hg.What would be the vapour pressure of the solution?
(b) (i) (1)What is Hinsberg's reagent?
(2) Arrange the following compounds in the increasing order of their basic strength in gaseous phase:
C2H5.NH2.(C2H5)2.NH
(ii) Give reasons for the following:
(i) Methyl amine is more basic than aniline.
(ii) Aniline readily reacts with bromine water to give 2,4,6-tribromoaniline
(iii) Primary amines have higher boiling points than tertiary amines
OR
(b) (i) Predict whether Van't Hoff factor will be less or greater than one,when ethanoic acid is dissolved in benzene
(ii) Define ideal solution
(iii) Calculate the mass of CaCl2(molar mass=111 gmol-1) to the dissolved in 500g of water to lower its freezing point by 2K,assuming that CaCl2 undergoes complete dissociation
(Kf for water=1.86 K kg mol-1)
(Kf for water=1.86 K kg mol-1)
Solution
 ,
,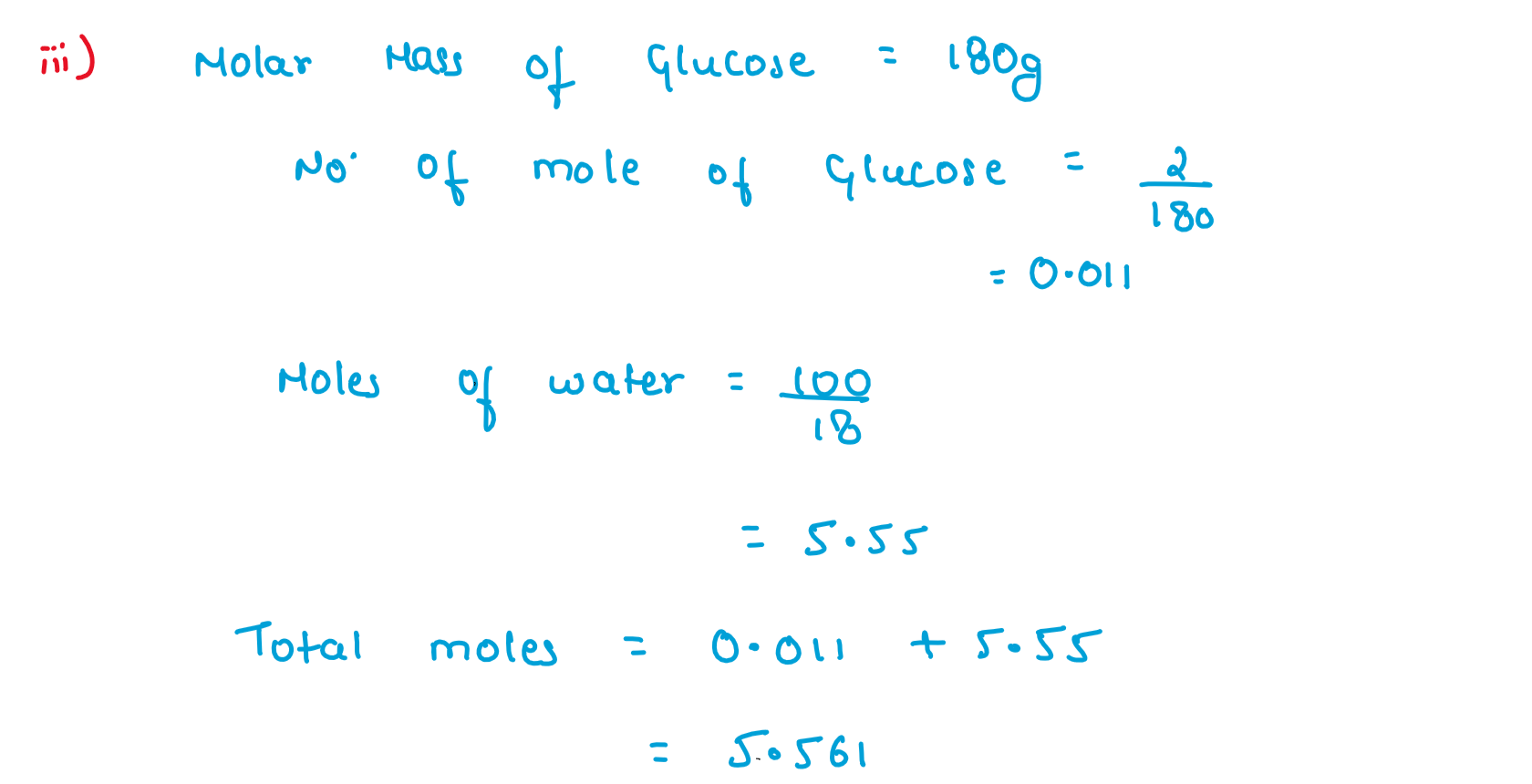 ,
,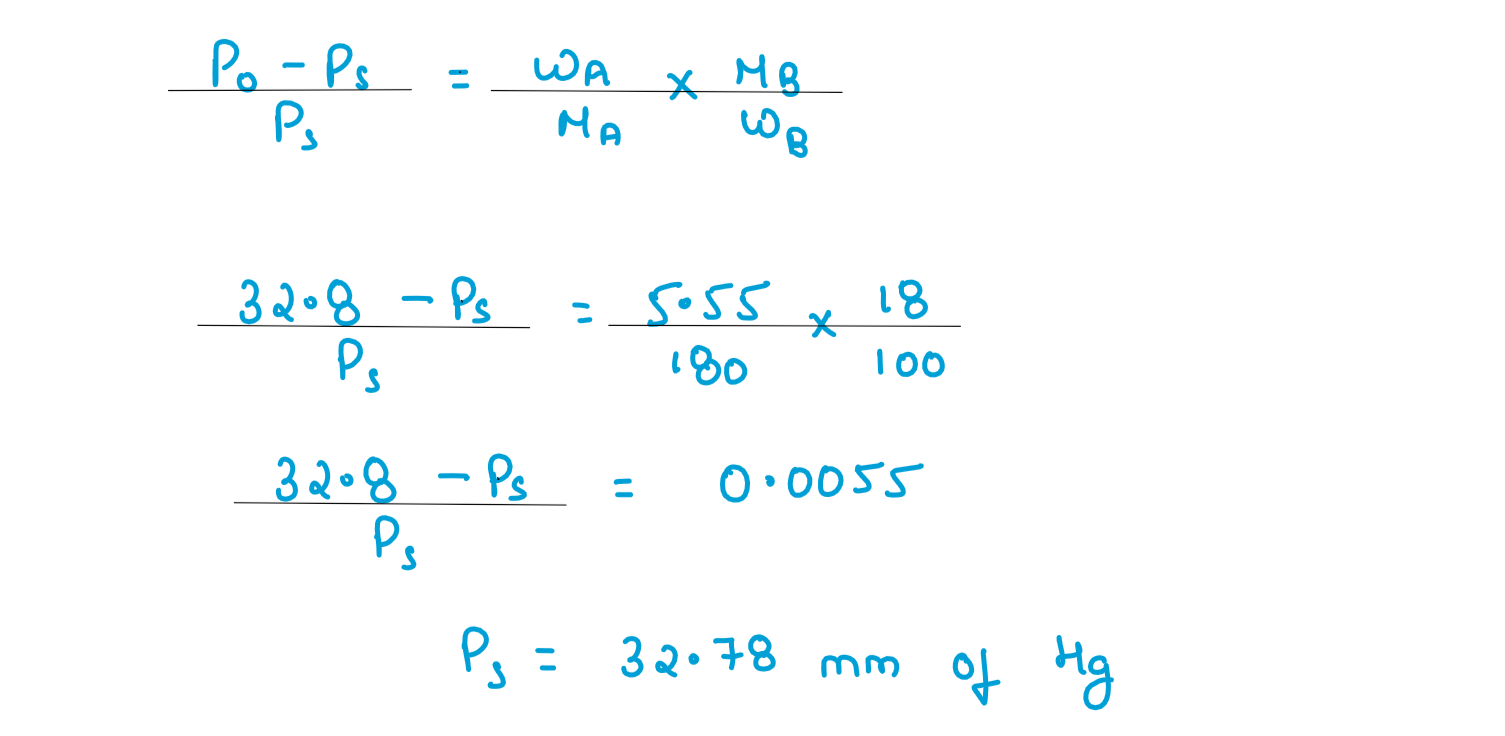 ,
, ,
,