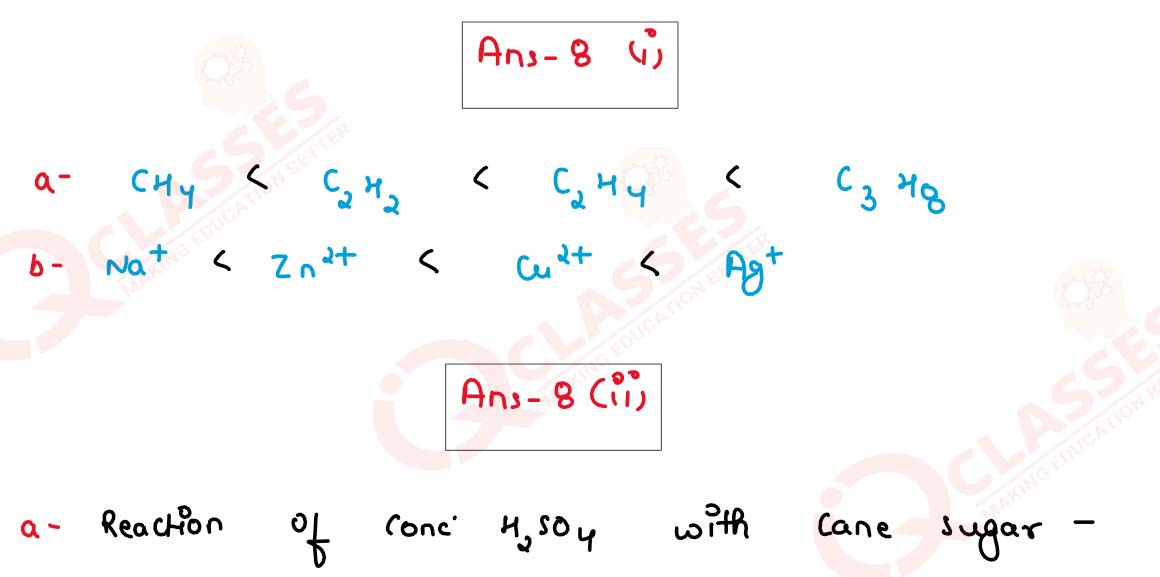
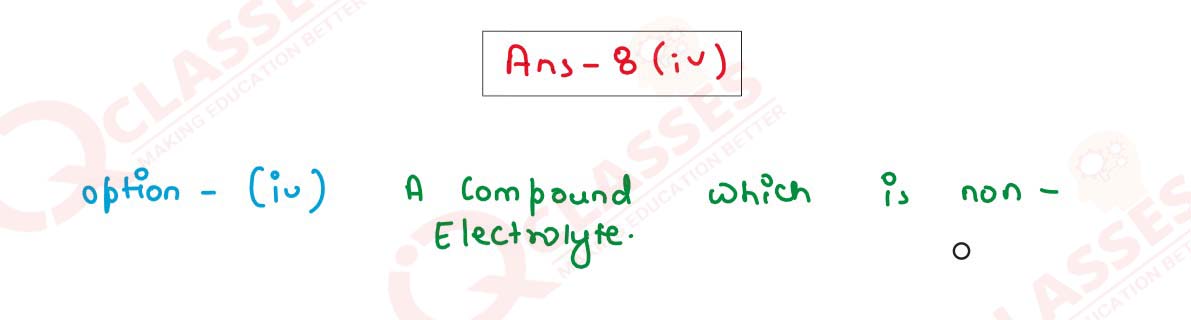(i) Arrange the following according to the instructions given in bracket
(a) C2H2, CH6, CH4, C2H4 (In the increasing order of the molecular weight)
(b) Cu2+, Na+, Zn2+, Ag+ (The order of Preferential discharge at the cathode)
(ii) Differentiate between the following pairs bused on the criteria given in the brackets:
(a) Cane sugar and hydrated copper sulphate [using concentrated H2SO4]
(b) Sulphuric acid and hydrochloric acid [type of salts formed]
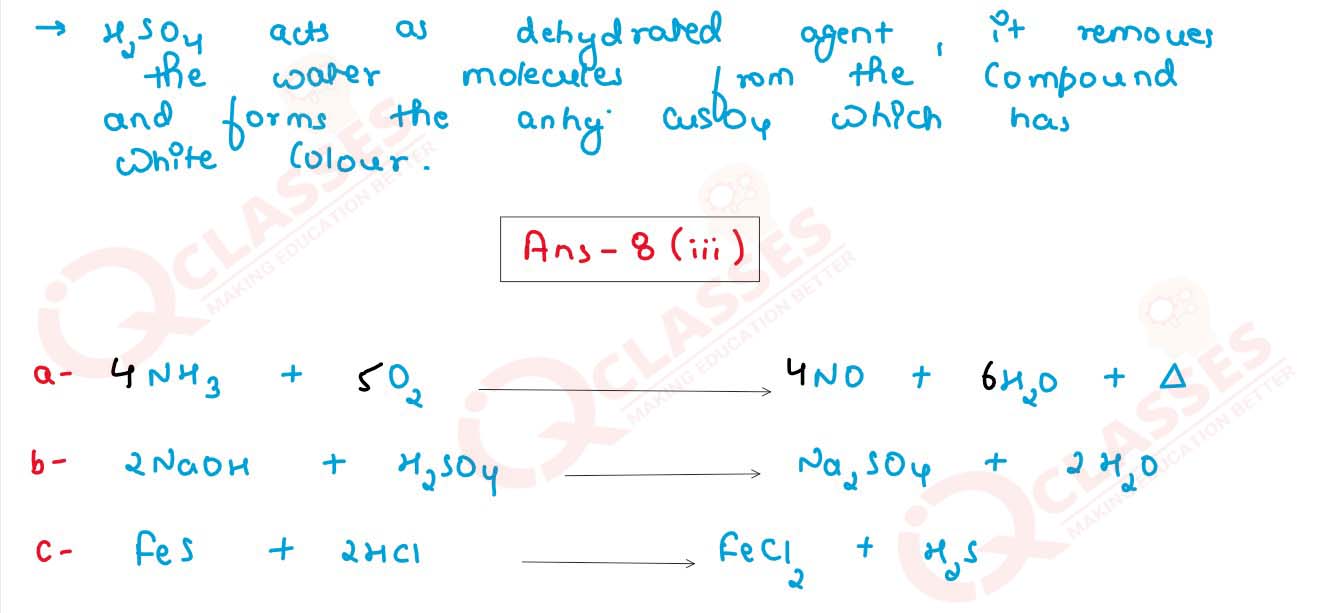
(iii) Convert the following reactions into a balanced chemical equation:
(a) Ammonia to nitric oxide using oxygen and platinum catalyst.
(b) Sodium hydroxide to sodium sulphate using sulphuric acid.
(c) Ferrous sulphide to hydrogen sulphide using hydrochloric acid
(iv) Choose the answer from the list which fits in the description:
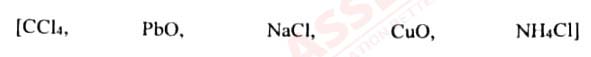
(a) A compound which undergoes thermal dissociation.
(b) An amphoteric oxide.
(c) A compound which is a non-electrolyte.
Solution
 ,
, ,
, ,
,