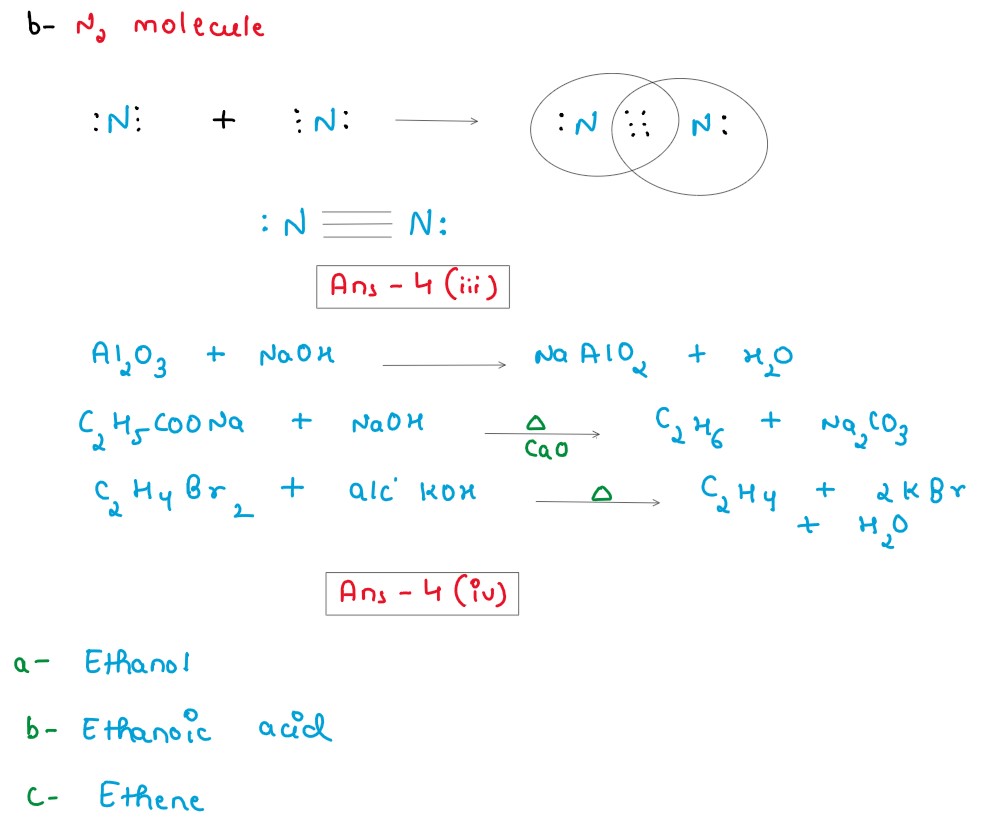
(i) Define the following terms:
(a) Molar volume
(b) Normal salt
(ii) Draw the electron dot structure of:
(a) Methane molecule
(b) Nitrogen molecule
[Atomic number: N=7,C= 6, H=1]
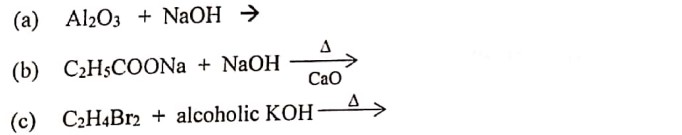
(iii) Complete and balance the following equations:
(iv) Choose the organic compound from the list given below to answer the following questions:
Ethene, Ethanoic acid, Ethanol, Methanal
(a) The compound which does not have a double bond in its structure.
(b) The compound which in its pure form turns into an ice like solid on cooling.
(c) The compound which is used for artificial ripening of fruits.
Solution
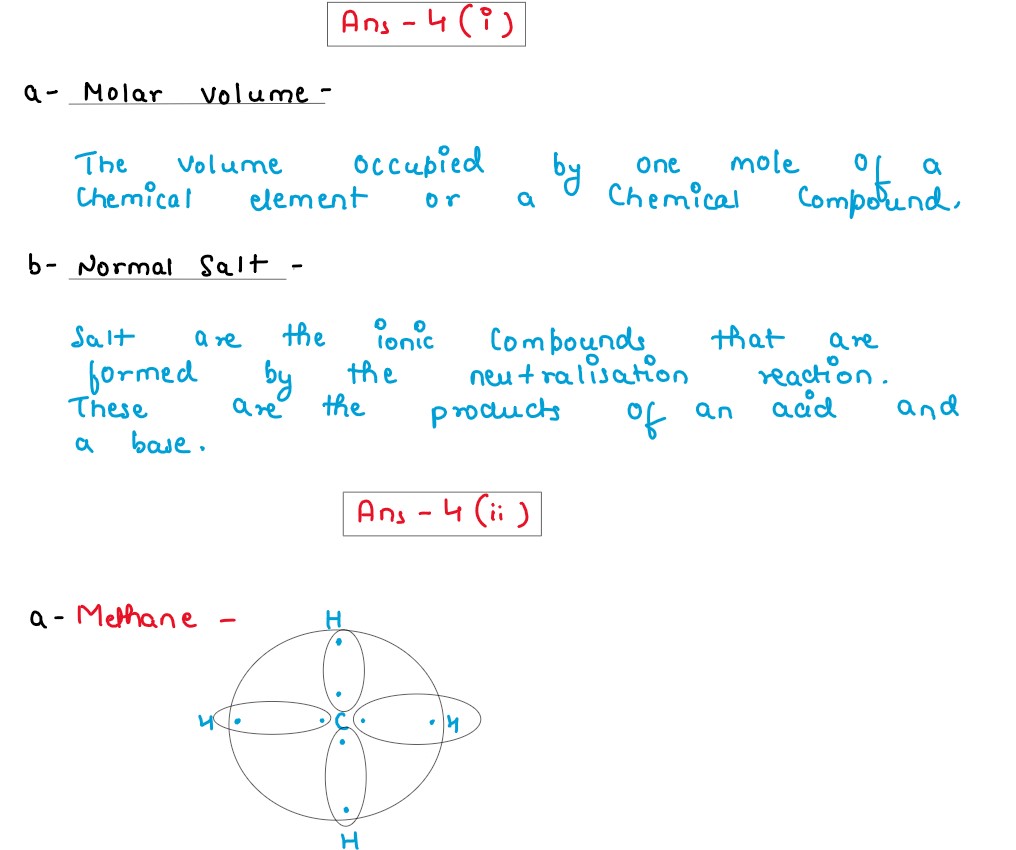 ,
,