(i) Element ‘X’ forms an oxide with the formula X203 which is a solid with high melting point. 'X' would most likely be placed in the group of the Periodic Table as:
1. (a) Na
(b) Mg
(c) Al
(d) Si
2. Justify your answer in the above question (1).
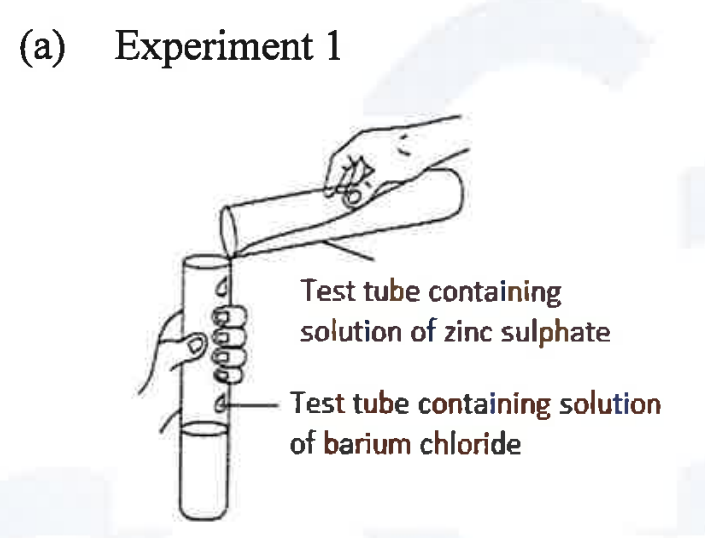
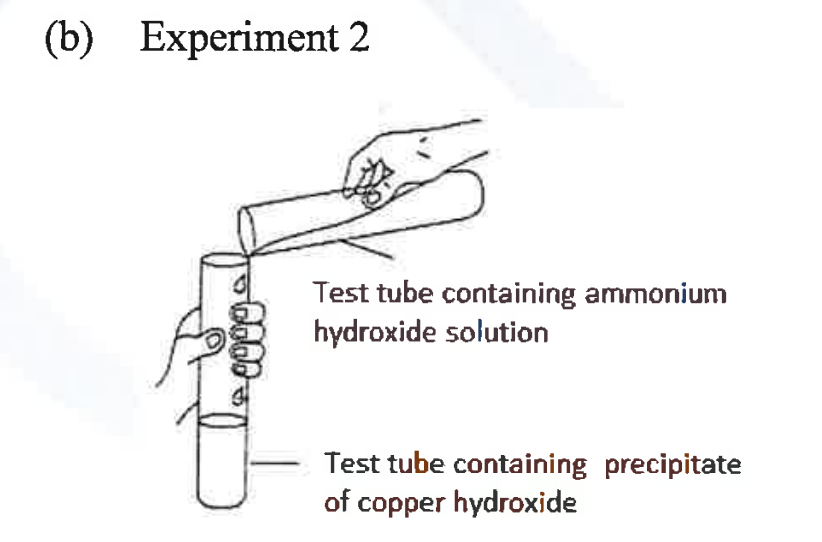
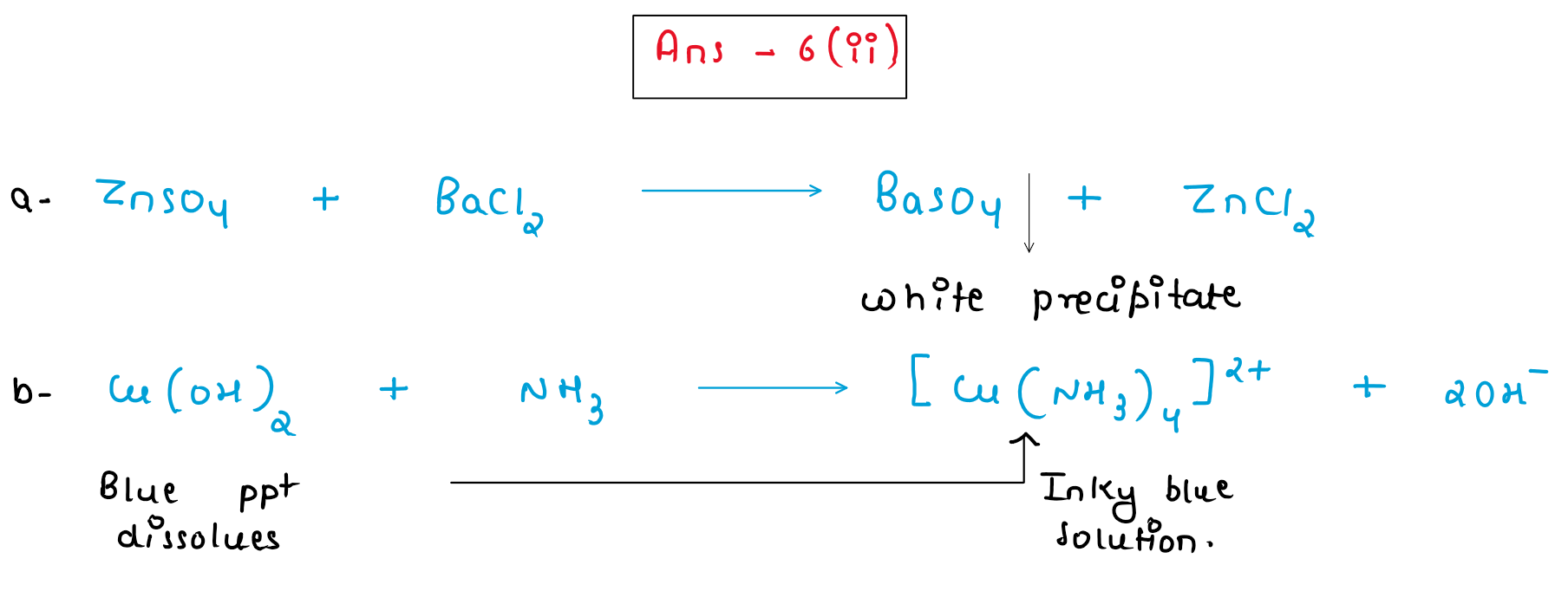
(ii) A student was asked to perform two experiments in the laboratory based [2] on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
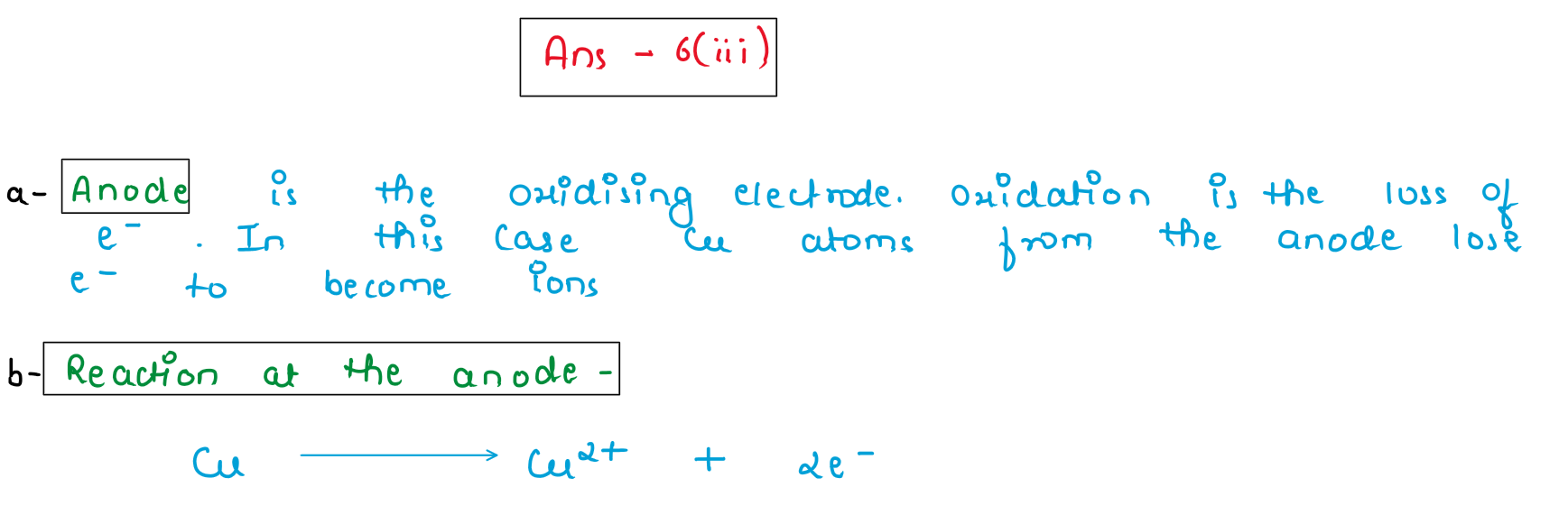
(iii) Copper sulphate solution is electrolysed using copper electrodes.
(a) Which electrode [cathode or anode] is the oxidizing electrode? Why?
(b) Write the equation for the reaction occurring at the above electrode.
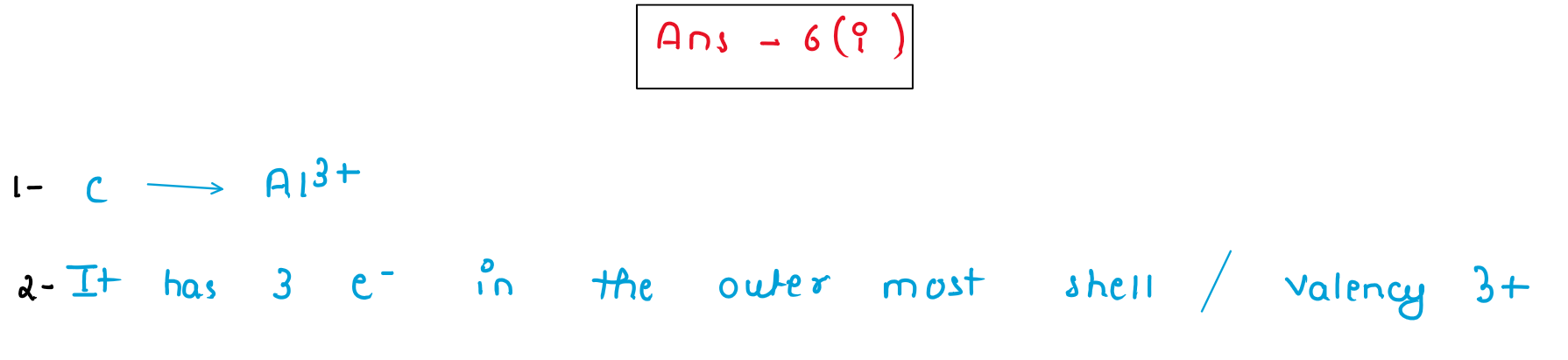
(iv) X [2, 8, 7] and Y [2, 8, 2] are two elements. Using this information complete the following:
(a) ______ is the metallic element.
(b) Metal atoms tend to have a maximum of ________ electrons in the outermost shell.
(c) ________ is the reducing agent.
Solution
 ,
, ,
, ,
,