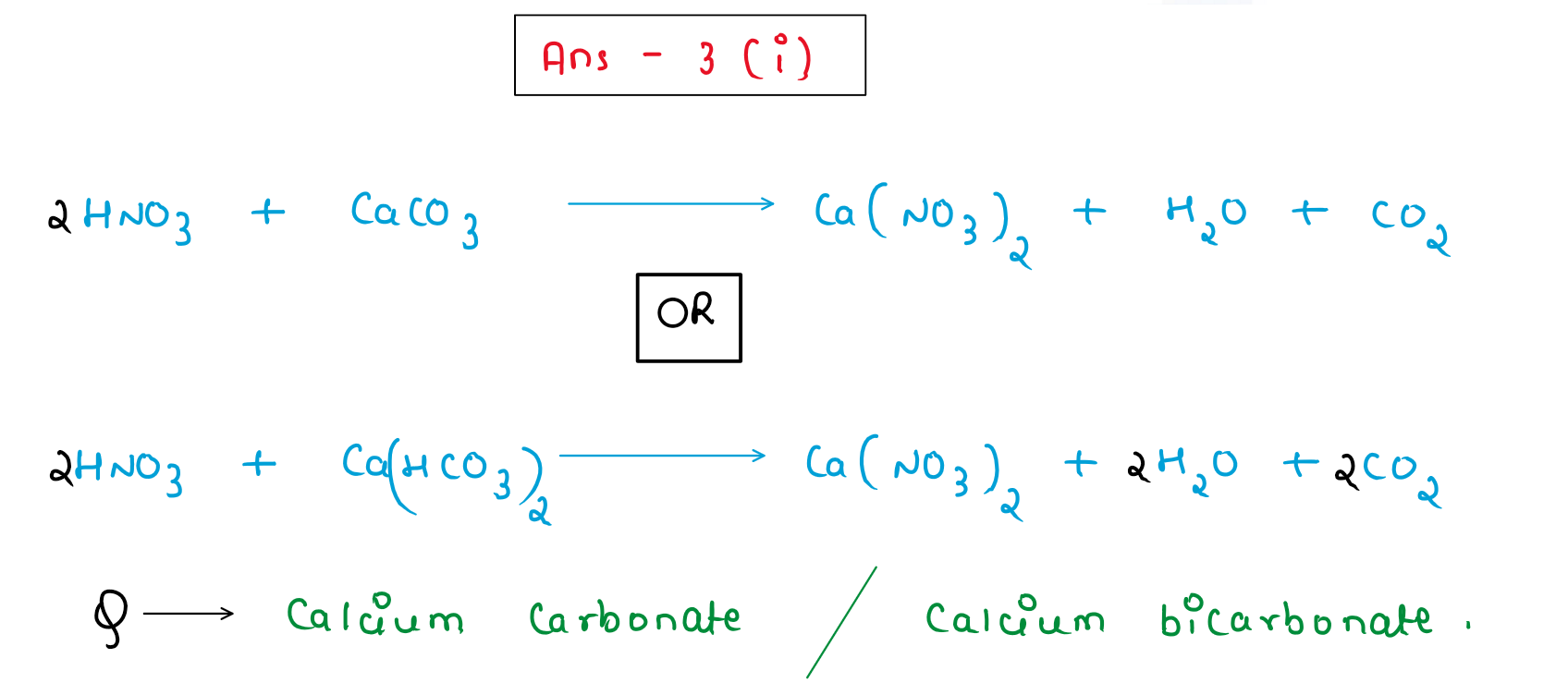
(i) Identify the reactant and write the balanced equation for the following:
Nitric acid reacts with compound Q to give a salt Ca(NO3)2, water and carbon dioxide.
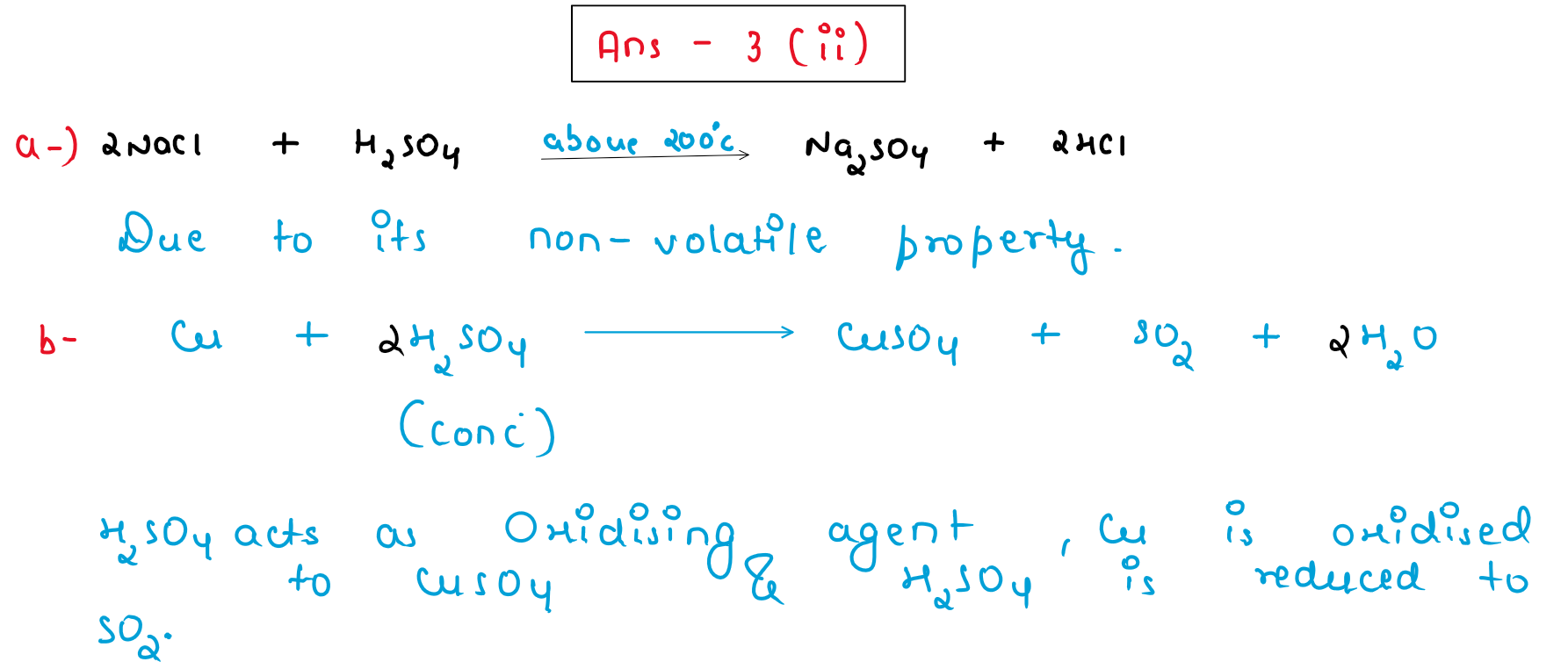
(ii) What property of Sulphuric acid is exhibited in each of the following cases:
(a) In the preparation of HCl gas when it reacts with Sodium chloride.
(b) When concentrated Sulphuric acid reacts with Copper to produce Sulphur dioxide gas.
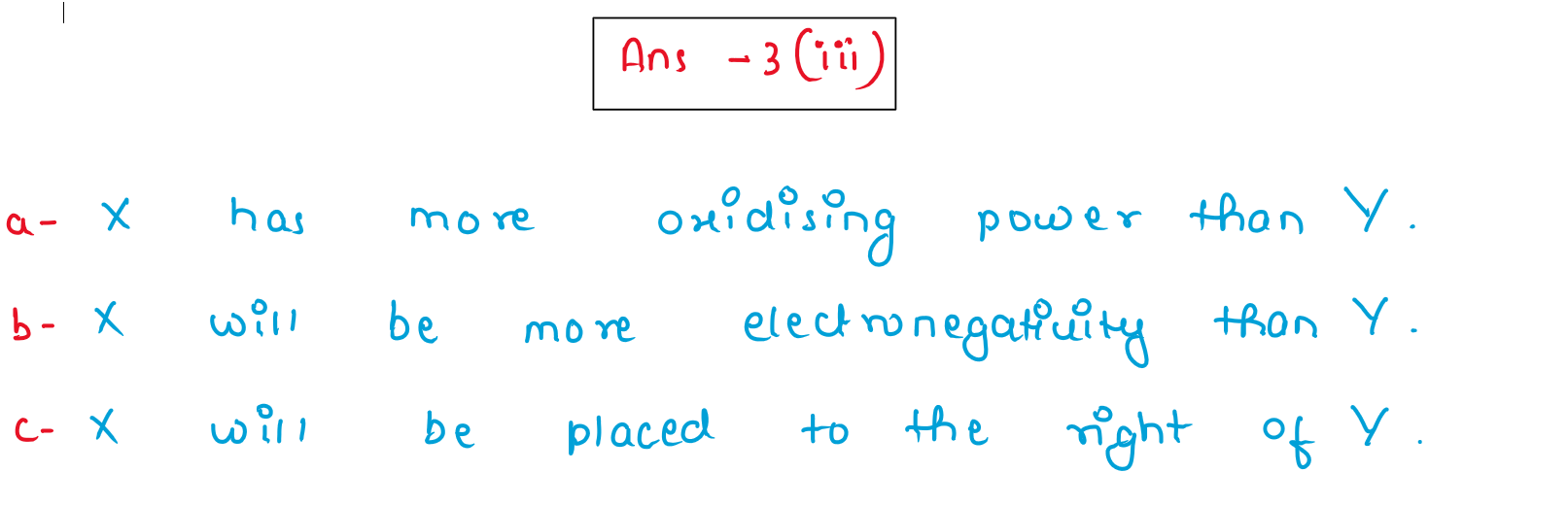
(iii) The electron affinity of an element X is greater than that of element Y.
(a) How is the oxidising power of X likely to compare with that of Y?
(b) How is the electronegativity of X likely to compare with that of Y?
(c) State whether X is likely to be placed to the left or to the right of Y in the periodic table?
(iv) You are provided with the list of chemicals mentioned below in the box:
Using suitable chemicals from the list given, write balanced chemical equation for the preparation of the salts mentioned below:
(a) copper sulphate
(b) sodium zincate
(c) ferric chloride
Solution
 ,
, ,
, ,
,