(i) Rita was given an unknown salt for identification. She prepared a solution of the salt [2] and divided it into two parts.
- To the first part of the salt solution, she added a few drops of ammonium hydroxide and obtained a reddish-brown precipitate.
- To the second part of the salt solution, she added a few drops of silver nitrate solution and obtained a white precipitate.
Name:
(a) the cation present and
(b) the anion present in the salt given for identification.
(ii) Fill in the blanks by choosing the correct answer from the bracket:
(a) Carbon tetrachloride is a _____ [polar / non-polar ] covalent molecule.
(b) During electrolysis of acidulated water, the gas liberated at the anode is ____ [oxygen / hydrogen].
(iii) Ammonia burns in Oxygen as shown below.
4NH3 + 3O2 → 2N2 + 6H2O
If 240 cc of ammonia is burnt in 300 cc of oxygen, find out the composition of the resultant gaseous mixture at room temperature.
(iv) The following table shows the electronic configuration of the atoms A, B, C and D.
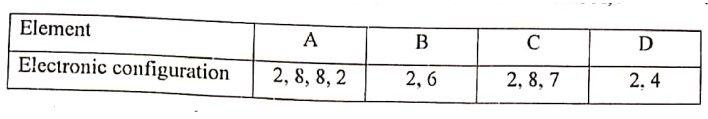
(a) Write the formula of the compound formed between:
1. A and B
2. D and C
(b) Which of the above elements will exhibit catenation?
Solution
 ,
,