1
Write the probable colour of the following salts :
(a) Ferrous salts
(b) Ammonium salts
(c) Cupric salts
(d) Calcium salts
(e) Aluminium salts.
Solution
(a) Ferrous salts
(b) Ammonium salts
(c) Cupric salts
(d) Calcium salts
(e) Aluminium salts.
Solution

2
Name:
(a) a metallic hydroxide soluble in excess of NH4OH.
(b) a metallic oxide soluble in excess of caustic soda solution.
(c) a strong alkali.
(d) a weak alkali.
(e) two colourless metal ions.
(f) two coloured metal ions.
(g) a metal that evolves a gas which burns with a pop sound when boiled with alkali solutions.
(h) two bases which are not alkalis but dissolve alkalis.
(i) a coloured metallic oxide which dissolve in strong alkalis to yield colourless solutions.
(j) a colourless cation not a representative element.
Solution

(a) a metallic hydroxide soluble in excess of NH4OH.
(b) a metallic oxide soluble in excess of caustic soda solution.
(c) a strong alkali.
(d) a weak alkali.
(e) two colourless metal ions.
(f) two coloured metal ions.
(g) a metal that evolves a gas which burns with a pop sound when boiled with alkali solutions.
(h) two bases which are not alkalis but dissolve alkalis.
(i) a coloured metallic oxide which dissolve in strong alkalis to yield colourless solutions.
(j) a colourless cation not a representative element.
Solution


3
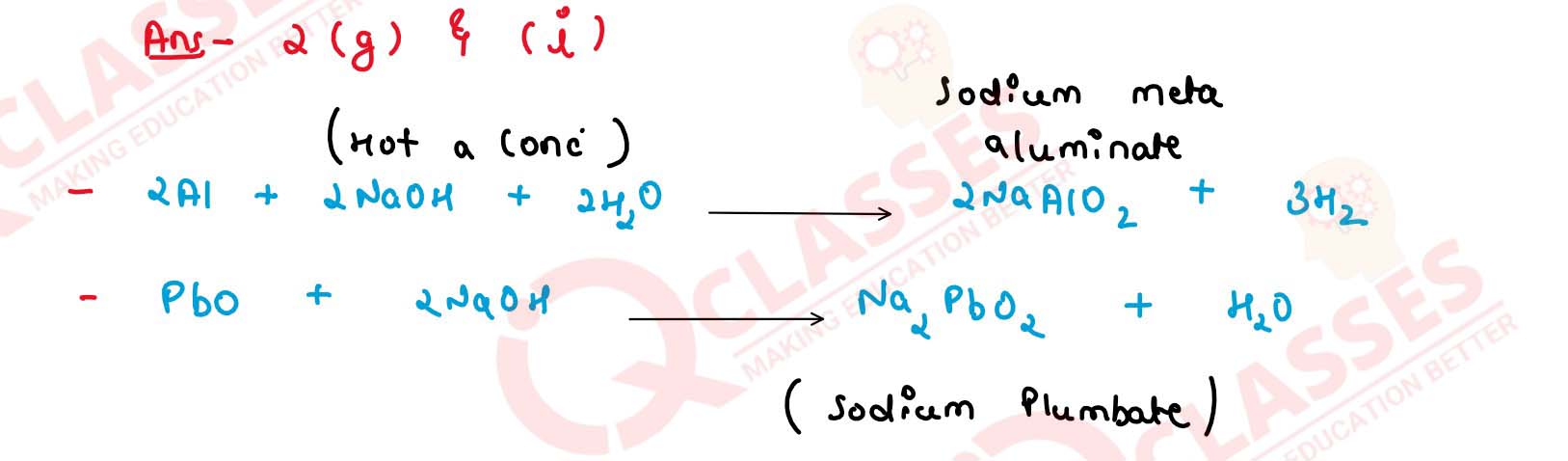
Write balanced equations for Q.2 (g) and (i).
Solution

4
What happens when ammonia solution is added first dropwise and then in excess to the following solutions
:
(i) CuSO4
(ii) ZnSO4
(iii) FeCl3
Write balanced equations for these reactions. Solution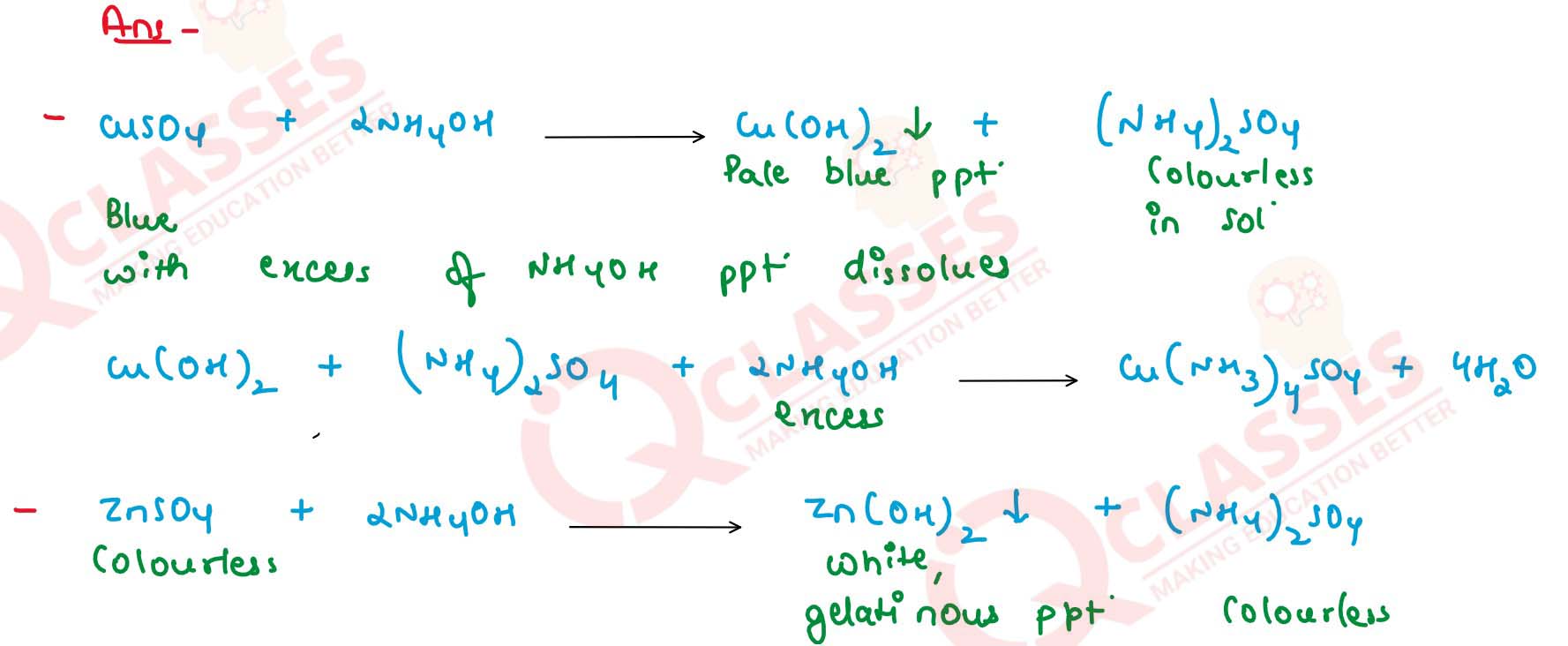
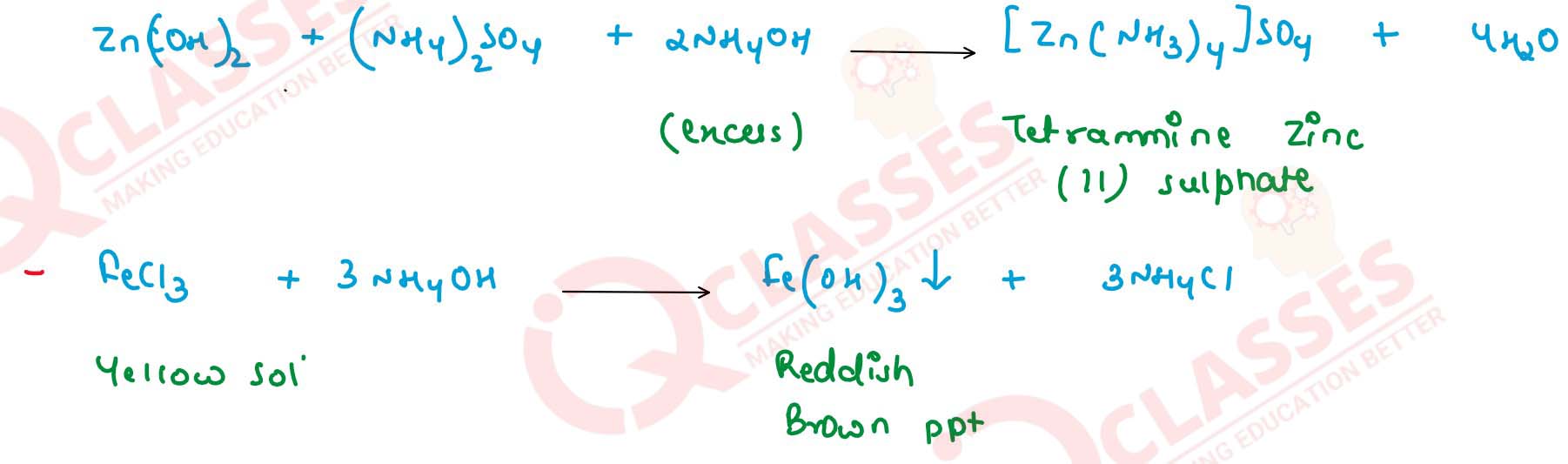
(i) CuSO4
(ii) ZnSO4
(iii) FeCl3
Write balanced equations for these reactions. Solution


5
What do you observe when caustic soda solution is added to the following solution, first a little and
then in excess:
(a) FeCl3
(b) ZnSO4
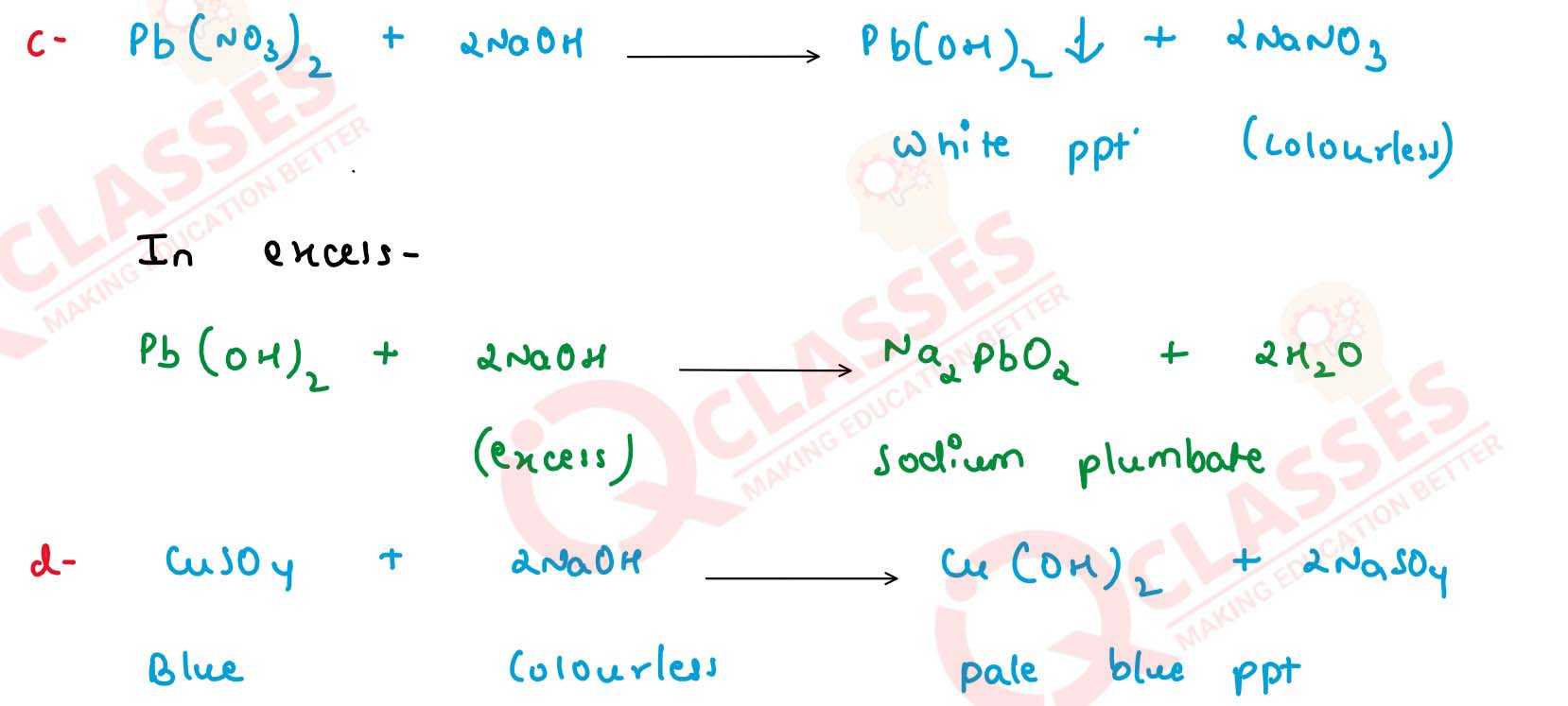
(c) Pb(NO3)2
(d) CuSO4
Write balanced equations for these reactions. Solution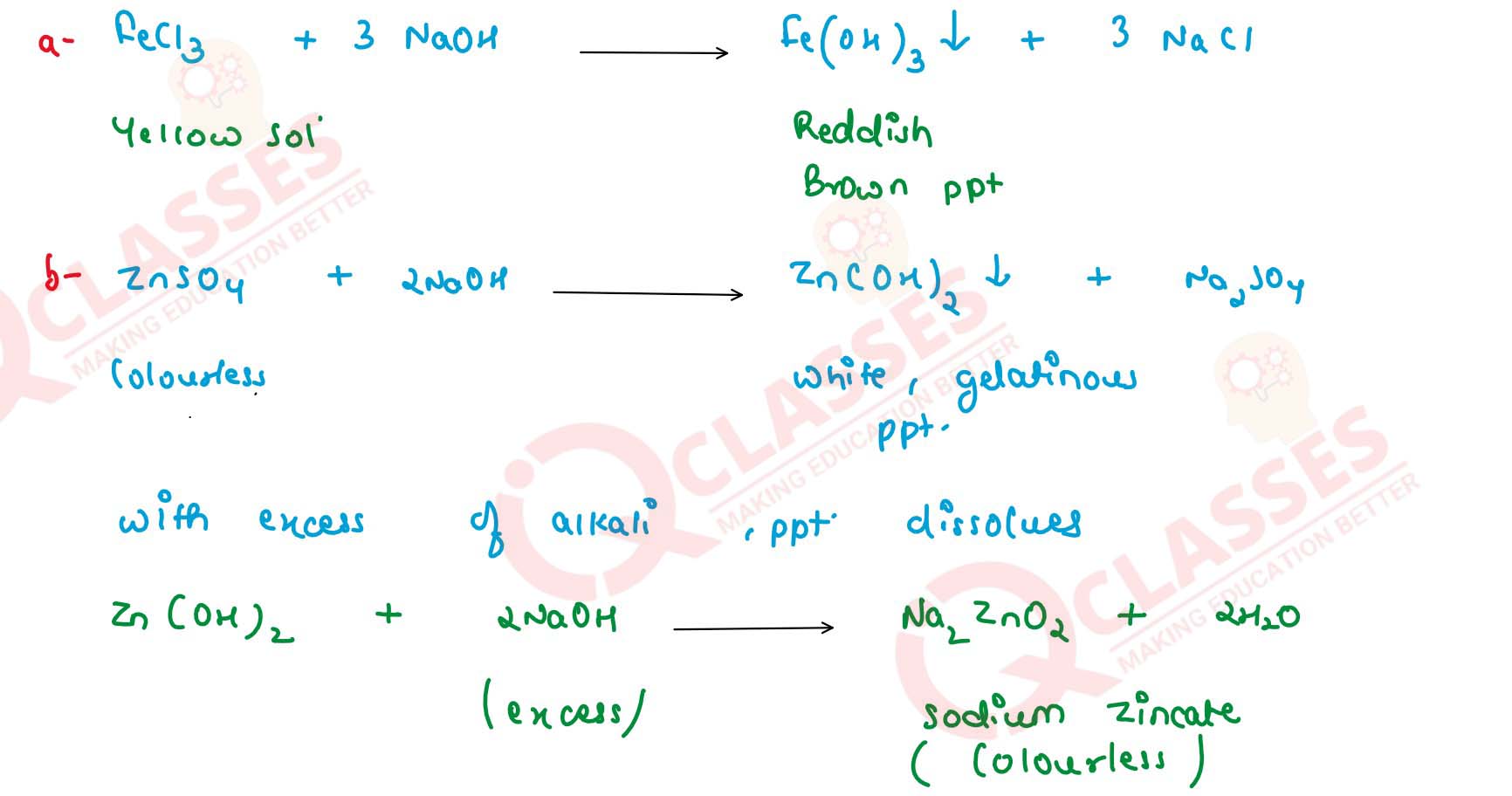
(a) FeCl3
(b) ZnSO4
(c) Pb(NO3)2
(d) CuSO4
Write balanced equations for these reactions. Solution


6
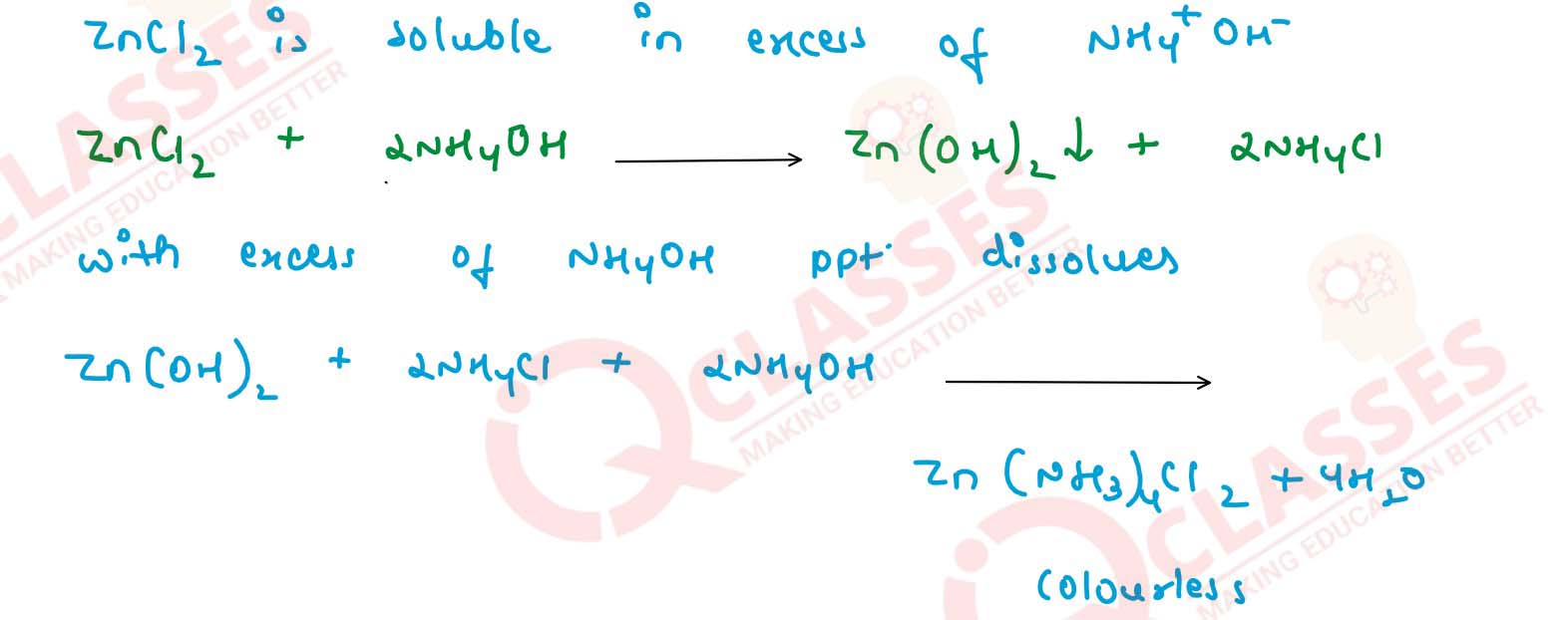
Name the chloride of a metal which is soluble in excess of ammonium hydroxide. Write equation for the
same.
Solution

7
On adding dilute ammonia solution to a colourless solution of a salt, a white gelatinous precipitate
appears. This precipitate however dissolves on addition of excess of ammonia solution. Identify (choose
from Na, Al, Zn, Pb, Fe)
(a) Which metal salt solution was used ?
(b) What is the formula of the white gelatinous precipitate obtained ?
Solution
(a) Which metal salt solution was used ?
(b) What is the formula of the white gelatinous precipitate obtained ?
Solution

8
(a) a yellow monoxide that dissolves in hot and concentrated caustic alkali.
(b) a white, insoluble oxide that dissolves when fused with caustic soda or caustic potash.
(c) a compound containing zinc in the anion.
Solution
(b) a white, insoluble oxide that dissolves when fused with caustic soda or caustic potash.
(c) a compound containing zinc in the anion.
Solution

9
Select the correct answers -
(a) Colour of an aqueous solution of copper sulphate is
(i) Green
(ii) Brown
(iii) Blue
(iv) Yellow
(b) Colour of the precipitate formed on adding NaOH solution to iron (II) sulphate solution is
(i) White
(ii) Brown
(iii) Green
(iv) Pale blue
(c) A metal which produces hydrogen on reacting with alkali as well as with acid.
(i) Iron
(ii) Magnesium
(iii) Zinc
(iv) Copper
(d) The salt solution which does not react with ammonium hydroxide is
(i) Calcium nitrate
(ii) Zinc nitrate
(iii) Lead nitrate
(iv) Copper nitrate (2018)
Solution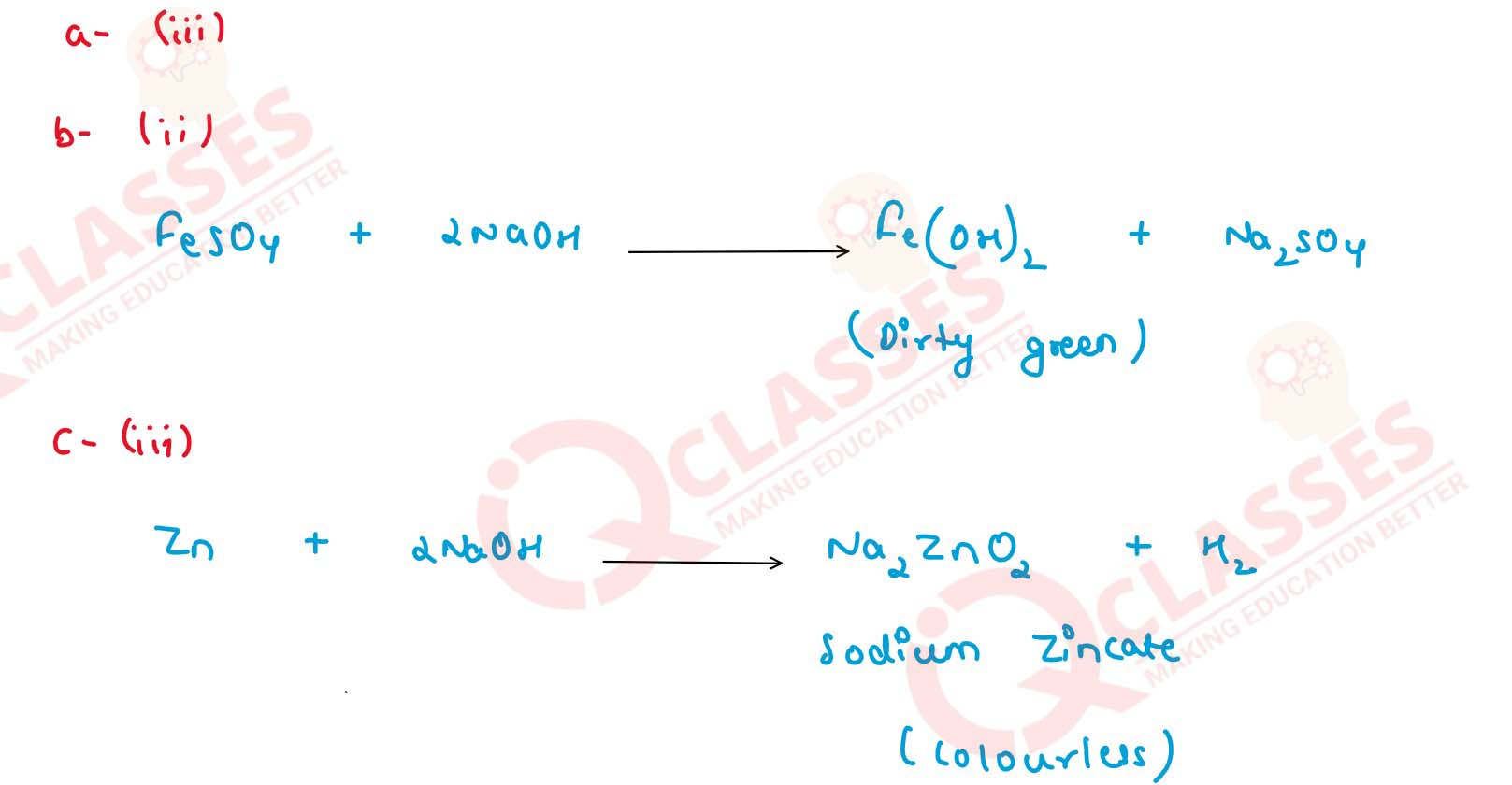
(a) Colour of an aqueous solution of copper sulphate is
(i) Green
(ii) Brown
(iii) Blue
(iv) Yellow
(b) Colour of the precipitate formed on adding NaOH solution to iron (II) sulphate solution is
(i) White
(ii) Brown
(iii) Green
(iv) Pale blue
(c) A metal which produces hydrogen on reacting with alkali as well as with acid.
(i) Iron
(ii) Magnesium
(iii) Zinc
(iv) Copper
(d) The salt solution which does not react with ammonium hydroxide is
(i) Calcium nitrate
(ii) Zinc nitrate
(iii) Lead nitrate
(iv) Copper nitrate (2018)
Solution

10
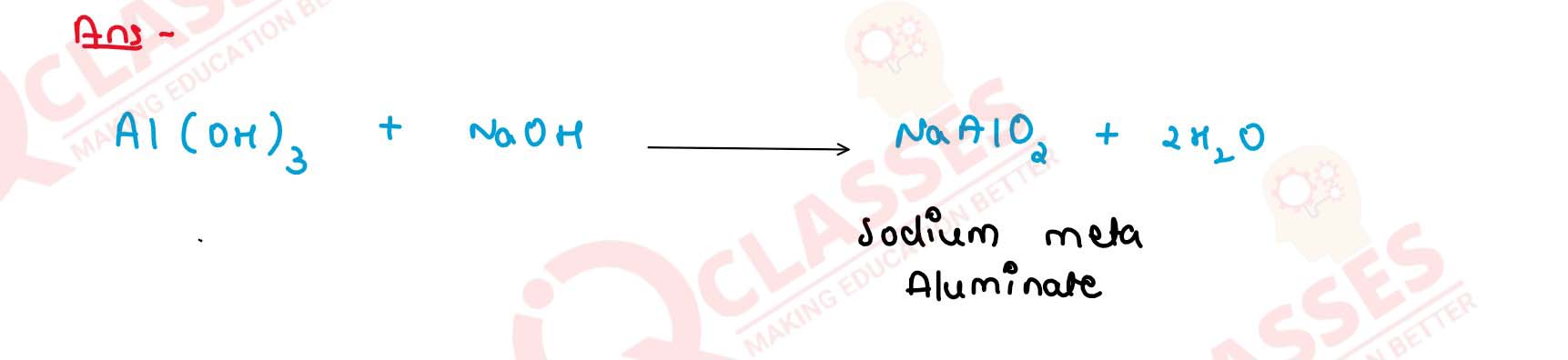
What do you observe when freshly precipitated
aluminium hydroxide reacts with caustic soda solution ?
Give balanced equation.
Solution

11
You are provided with two reagent bottles marked A and
B. One contains NH4OH solution and the other contains
NaOH solution. How will you identify them by a chemical
test ?
Solution
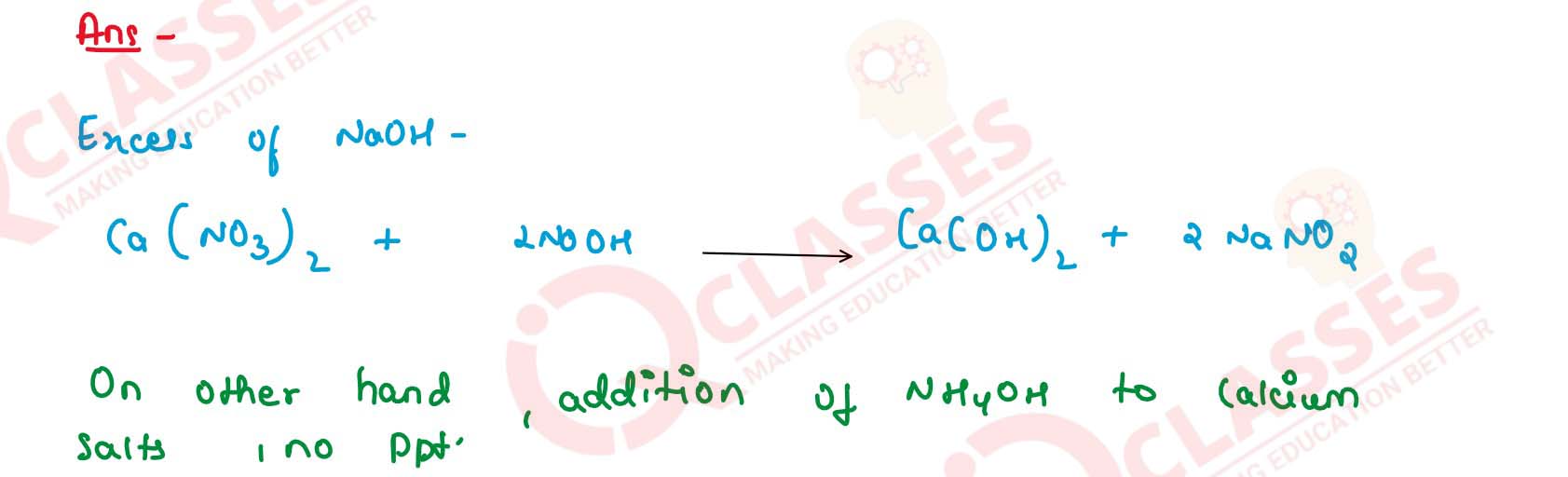

12
Distinguish by adding : Sodium hydroxide solution or
ammonium hydroxide solution to
(a) Calcium salt solution and lead salt solution.
(b) Lead nitrate solution and zinc nitrate solution.
(c) Copper salt solution and ferrous salt solution.
(d) Fe(II) salt solution and Fe(III) salt solution.
(e) Ferrous nitrate and lead nitrate
Solution

(a) Calcium salt solution and lead salt solution.
(b) Lead nitrate solution and zinc nitrate solution.
(c) Copper salt solution and ferrous salt solution.
(d) Fe(II) salt solution and Fe(III) salt solution.
(e) Ferrous nitrate and lead nitrate
Solution


13
How will you distinguish lead nitrate and zinc nitrate
solution ?
Solution


14
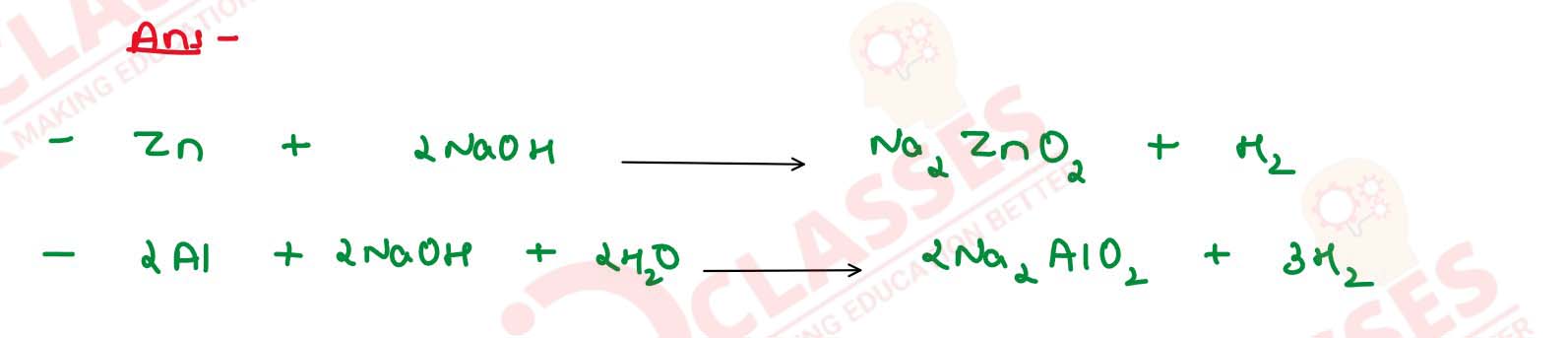
What is observed when hot concentrated caustic soda
solution is added to (a) Zinc and (b) Aluminium ? Write
balanced equations.
Solution

15
(a) What do you understand by amphoteric oxide ?
(b) Give the balanced equations for the reaction with two different amphoteric oxides with a caustic alkali.
(c) Name the products formed.
Solution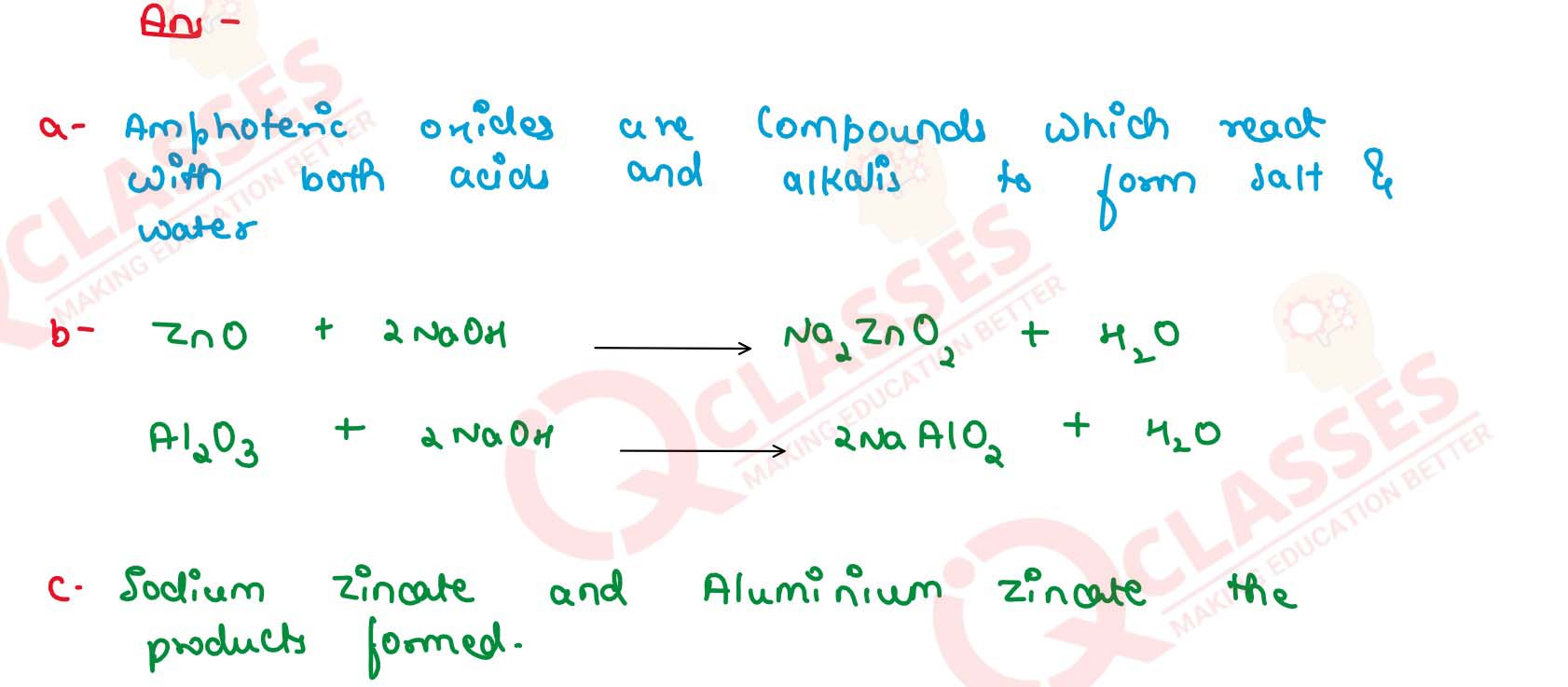
(b) Give the balanced equations for the reaction with two different amphoteric oxides with a caustic alkali.
(c) Name the products formed.
Solution

16
Write balanced equations for the following conversions
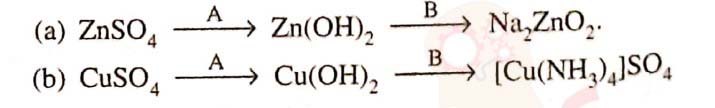 Solution
Solution
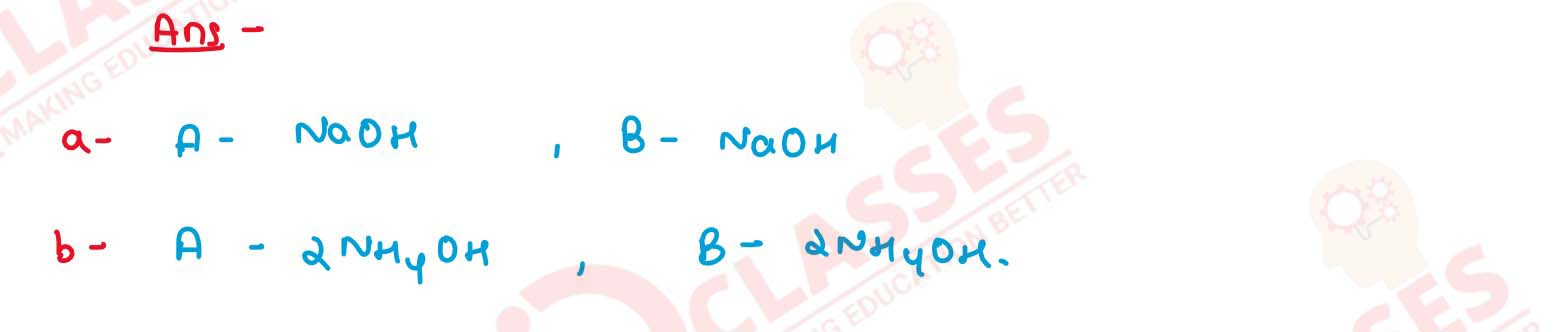
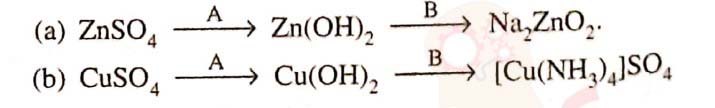 Solution
Solution
