1
Give reasons for the following :
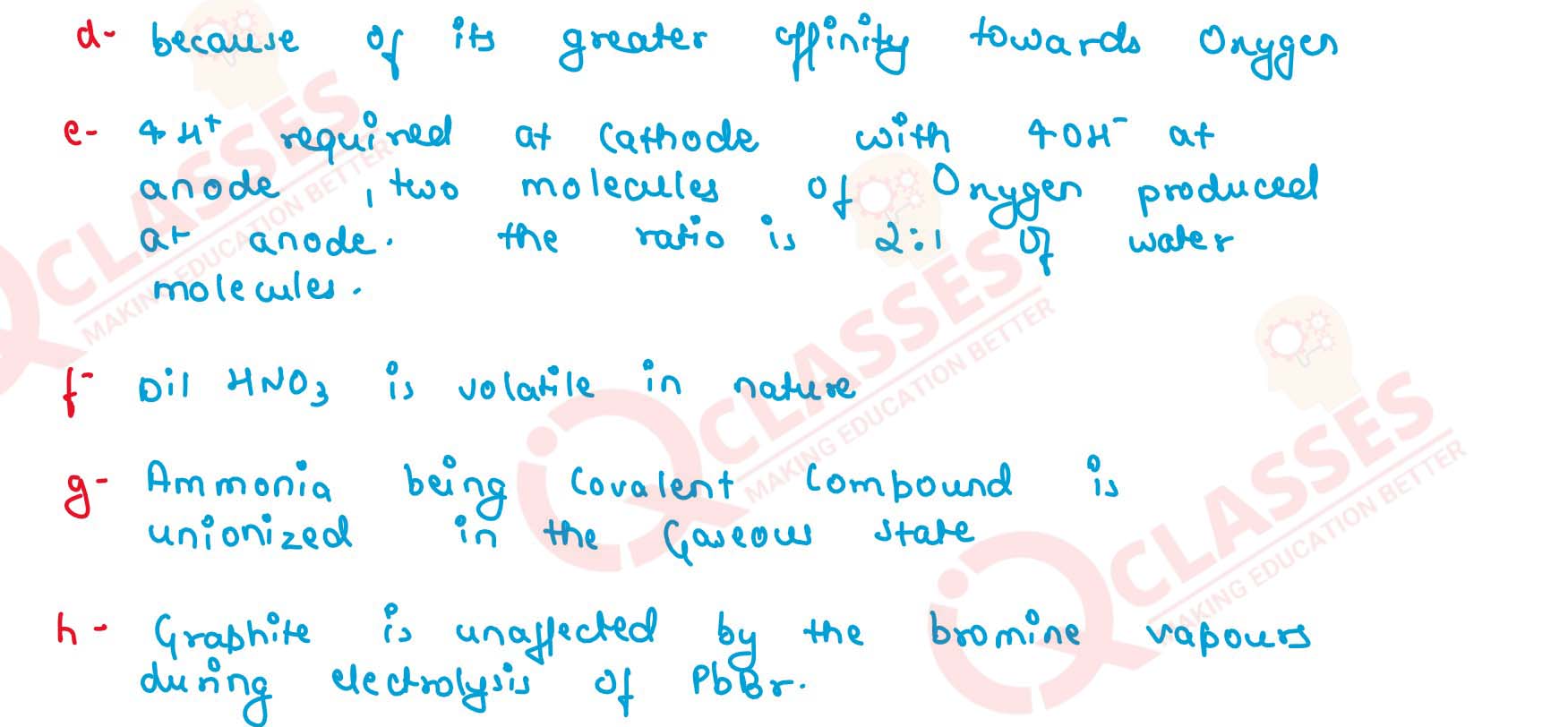
(a) Electrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction.
(b) The blue colour of aqueous copper sulphate fades when it is electrolysed using platinum electrodes.
(c) Lead bromide undergoes electrolytic dissociation in the molten state but is a non-electrolyte in the solid state.
(d) Aluminium is extracted from its oxide by electrolytic reduction and not by conventional reducing agents.
(e) The ratio of hydrogen and oxygen formed at the cathode and anode is 2 :1 by volume.
(f) In the electrolysis of acidified water, dilute sulphuric acid is preferred to dilute nitric acid for acidification.
(g) Ammonia is unionised in the gaseous state but in the aqueous solution, it is a weak electrolyte.
(h) A graphite anode is preferred to other inert electrodes during electrolysis of fused lead bromide.
(i) For electroplating with silver, silver nitrate is not used as electrolyte.
(j) Carbon tetrachloride is a liquid but does not conduct electricity.
(k) Potassium is not extracted by electrolysis of its aqueous salt solution.
Solution

(a) Electrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction.
(b) The blue colour of aqueous copper sulphate fades when it is electrolysed using platinum electrodes.
(c) Lead bromide undergoes electrolytic dissociation in the molten state but is a non-electrolyte in the solid state.
(d) Aluminium is extracted from its oxide by electrolytic reduction and not by conventional reducing agents.
(e) The ratio of hydrogen and oxygen formed at the cathode and anode is 2 :1 by volume.
(f) In the electrolysis of acidified water, dilute sulphuric acid is preferred to dilute nitric acid for acidification.
(g) Ammonia is unionised in the gaseous state but in the aqueous solution, it is a weak electrolyte.
(h) A graphite anode is preferred to other inert electrodes during electrolysis of fused lead bromide.
(i) For electroplating with silver, silver nitrate is not used as electrolyte.
(j) Carbon tetrachloride is a liquid but does not conduct electricity.
(k) Potassium is not extracted by electrolysis of its aqueous salt solution.
Solution



2
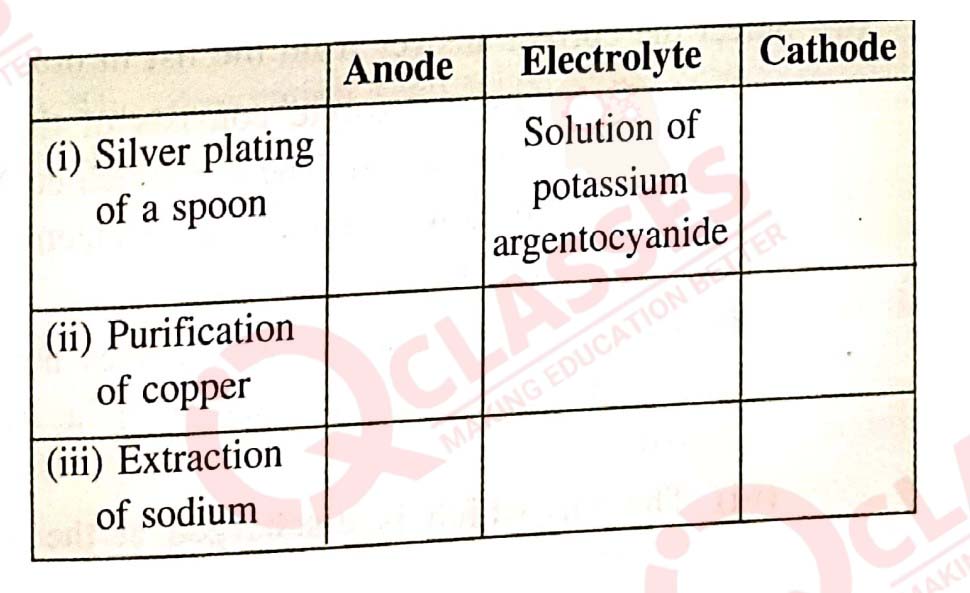
(a) Copy and complete the following table which refers to two
practical applications of electrolysis
(b) Write equations of reactions taking place at anode for Q.2(a). (2015)
Solution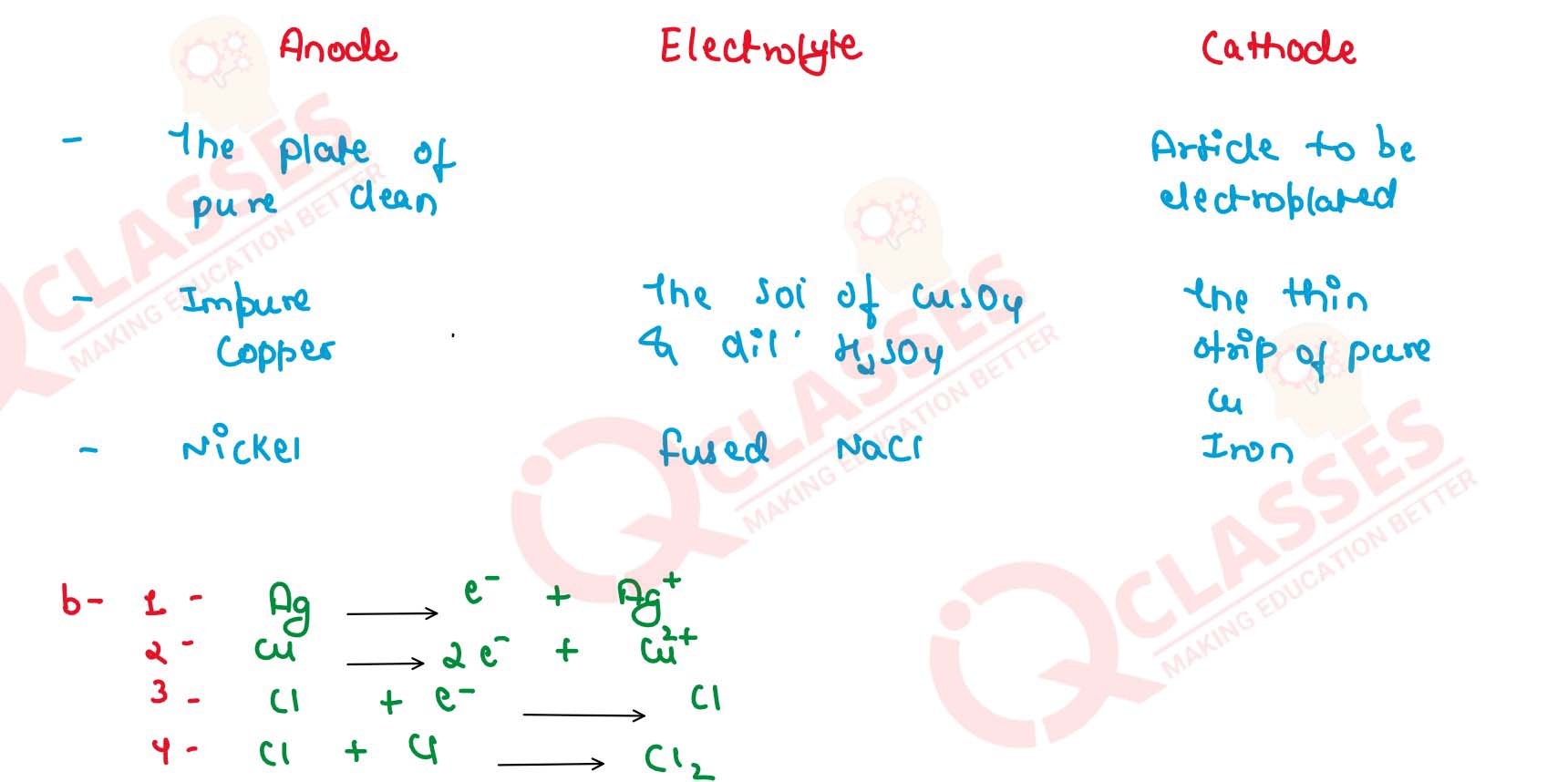

(b) Write equations of reactions taking place at anode for Q.2(a). (2015)
Solution

3
(a) Draw a labelled diagram to show how iron is electroplated with copper.
(b) Which solution is preferred as electrolyte, CuSO4, or FeSO4 ?
(c) Describe what happens to the iron object and the copper rod.
Solution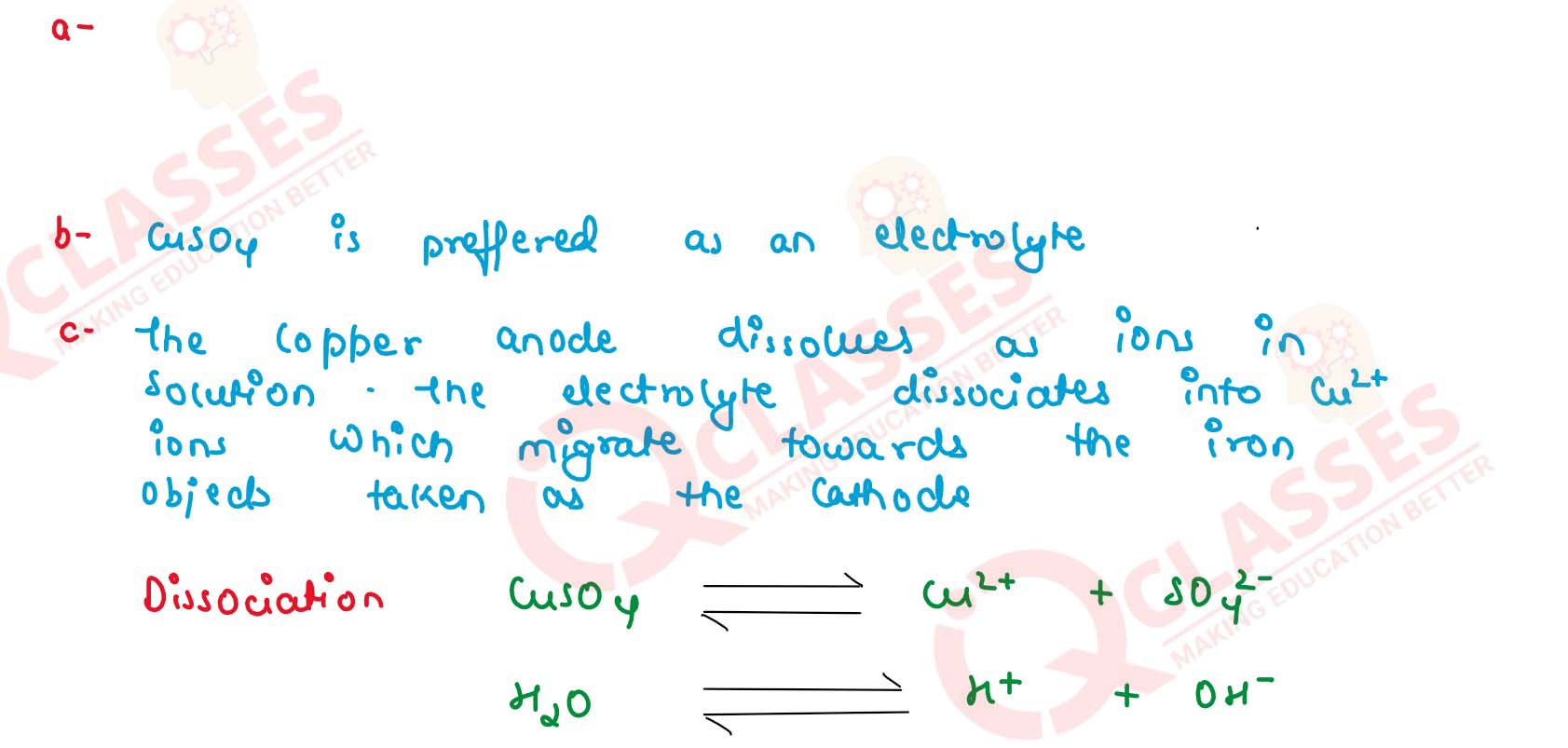
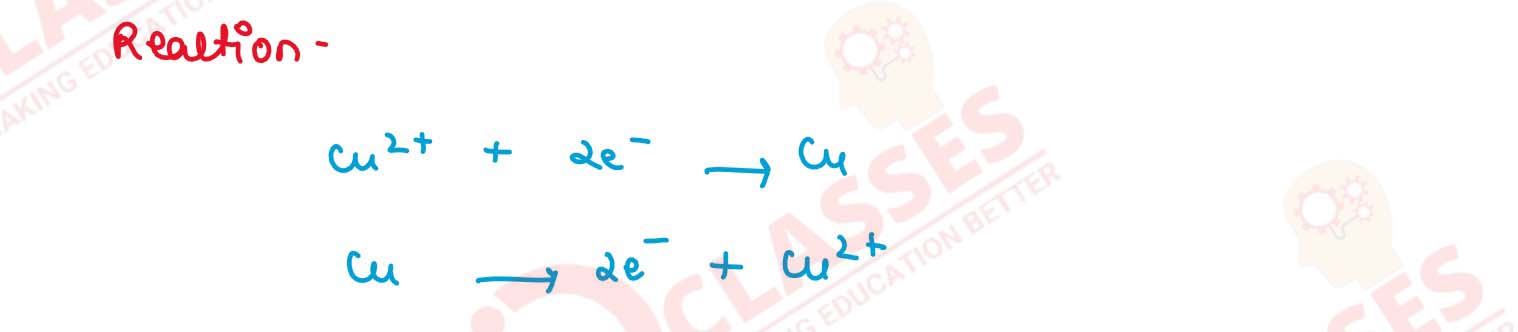
(b) Which solution is preferred as electrolyte, CuSO4, or FeSO4 ?
(c) Describe what happens to the iron object and the copper rod.
Solution

4
Element X is a metal with a valency 2. Element Y is a
non-metal with a valency 3.
(a) Write equations to show how X and Y form ions ?
(b) If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound. (2015)
(c) If the compound formed between X and Y is melted and an electric current passed through the molten compound, the element X will be obtained at the .............. and Y at the ................ of the electrolytic cell. (Provide the missing words).
Solution
(a) Write equations to show how X and Y form ions ?
(b) If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound. (2015)
(c) If the compound formed between X and Y is melted and an electric current passed through the molten compound, the element X will be obtained at the .............. and Y at the ................ of the electrolytic cell. (Provide the missing words).
Solution

5
Write two applications of electrolysis in which the anode
diminishes in mass.
Solution


6
(a) What kind of particles will be found in a liquid
compound which is a non- electrolyte?
(b) If HX is a weak acid, what particles will be present in its dilute solution apart from those of water ?
(c) Cations are formed by .............. (loss/gain) of electrons and anions are formed by .......... (loss/gain) of electrons. (Choose the correct word to fill in the blanks).
(d) What ions must be present in a solution used for electroplating a particular metal ? Solution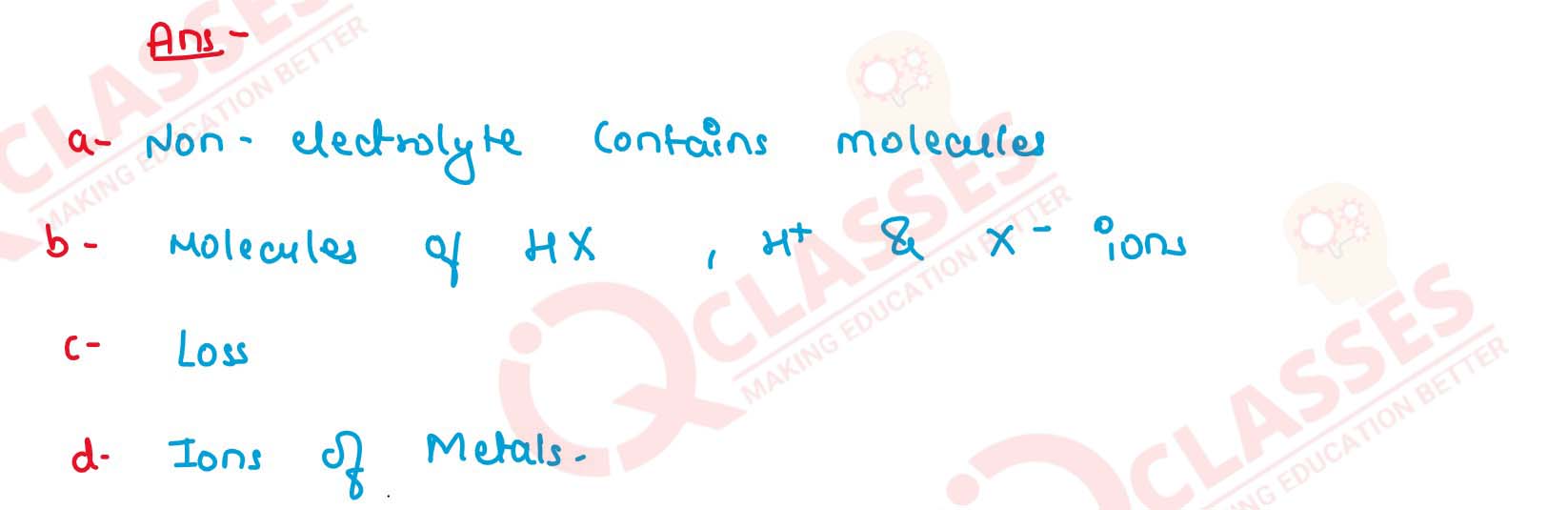
(b) If HX is a weak acid, what particles will be present in its dilute solution apart from those of water ?
(c) Cations are formed by .............. (loss/gain) of electrons and anions are formed by .......... (loss/gain) of electrons. (Choose the correct word to fill in the blanks).
(d) What ions must be present in a solution used for electroplating a particular metal ? Solution

7
A strip of copper is placed in four different colourless
salt solutions. They are KNO3, AgNO3 , Zn(NO3)2,
Ca(NO3)2. Which one of the solutions will finally turn
blue ?
Solution

