1
What is the significance of atomic number in the modern periodic table ?
Solution


2
Arrange the following as per instructions given in the brackets.
(a) Mg, Cl, Na, S, Si (decreasing order of atomic size)
(b) Cs, Na, Li, K, Rb (increasing metallic character)
(c) Na, K, Cl, S, Si (increasing ionisation potential)
(d) Cl, F, Br, I (increasing electron affinity)
(e) Cs, Na, Li, K, Rb (decreasing electronegativity)
(f) K, Pb, Ca, Zn (increasing reactivity)
(g) Li, K, Na, H (decreasing order of their potential difference)
Solution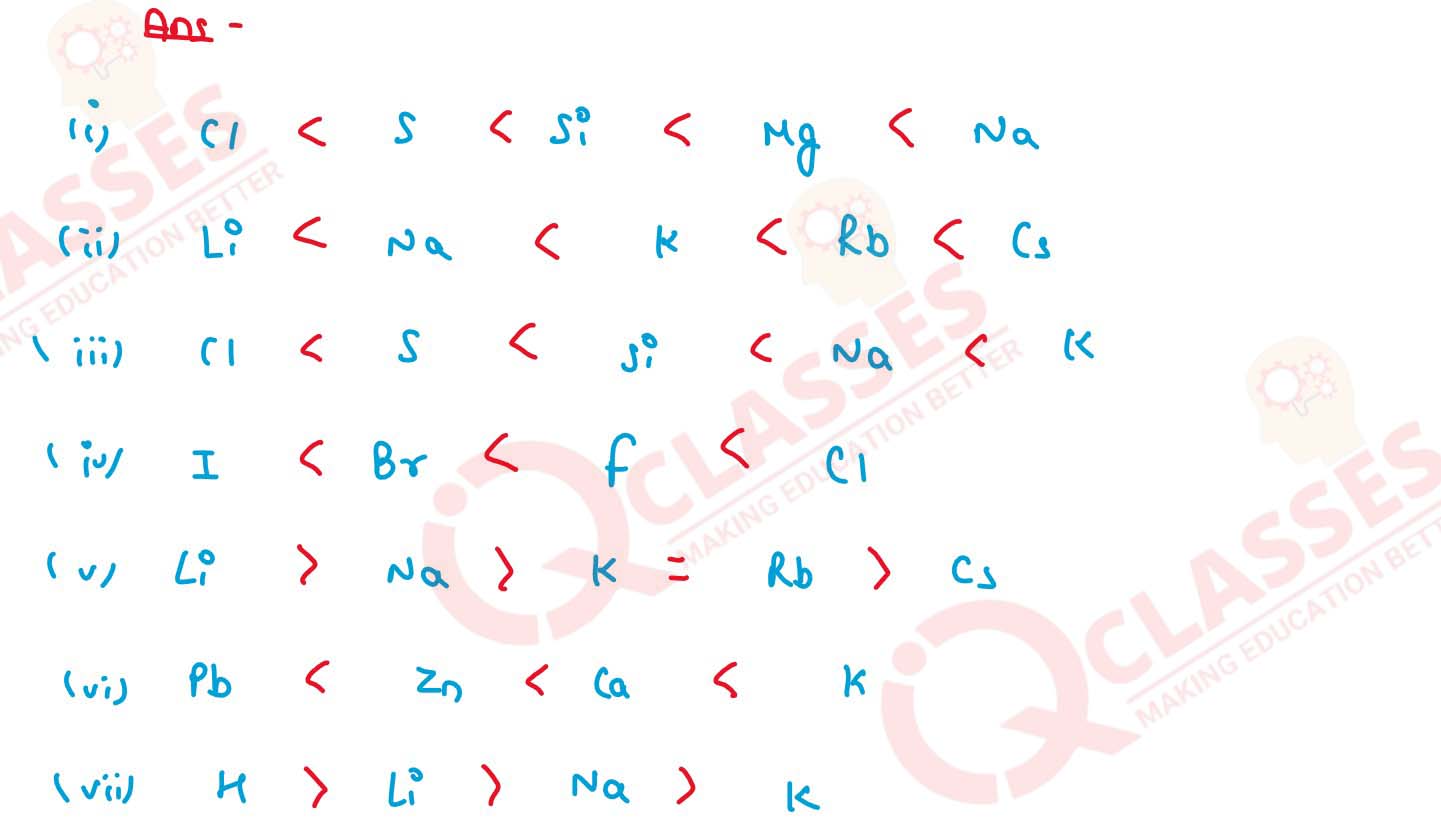
(a) Mg, Cl, Na, S, Si (decreasing order of atomic size)
(b) Cs, Na, Li, K, Rb (increasing metallic character)
(c) Na, K, Cl, S, Si (increasing ionisation potential)
(d) Cl, F, Br, I (increasing electron affinity)
(e) Cs, Na, Li, K, Rb (decreasing electronegativity)
(f) K, Pb, Ca, Zn (increasing reactivity)
(g) Li, K, Na, H (decreasing order of their potential difference)
Solution

3
Chlorine in the Periodic Table is surrounded by the
elements with atomic number 9, 16, 18 and 35.
(a) Which of these have Physical and Chemical properties resembling chlorine ?
(b) Which is more electronegative than chlorine ?
Solution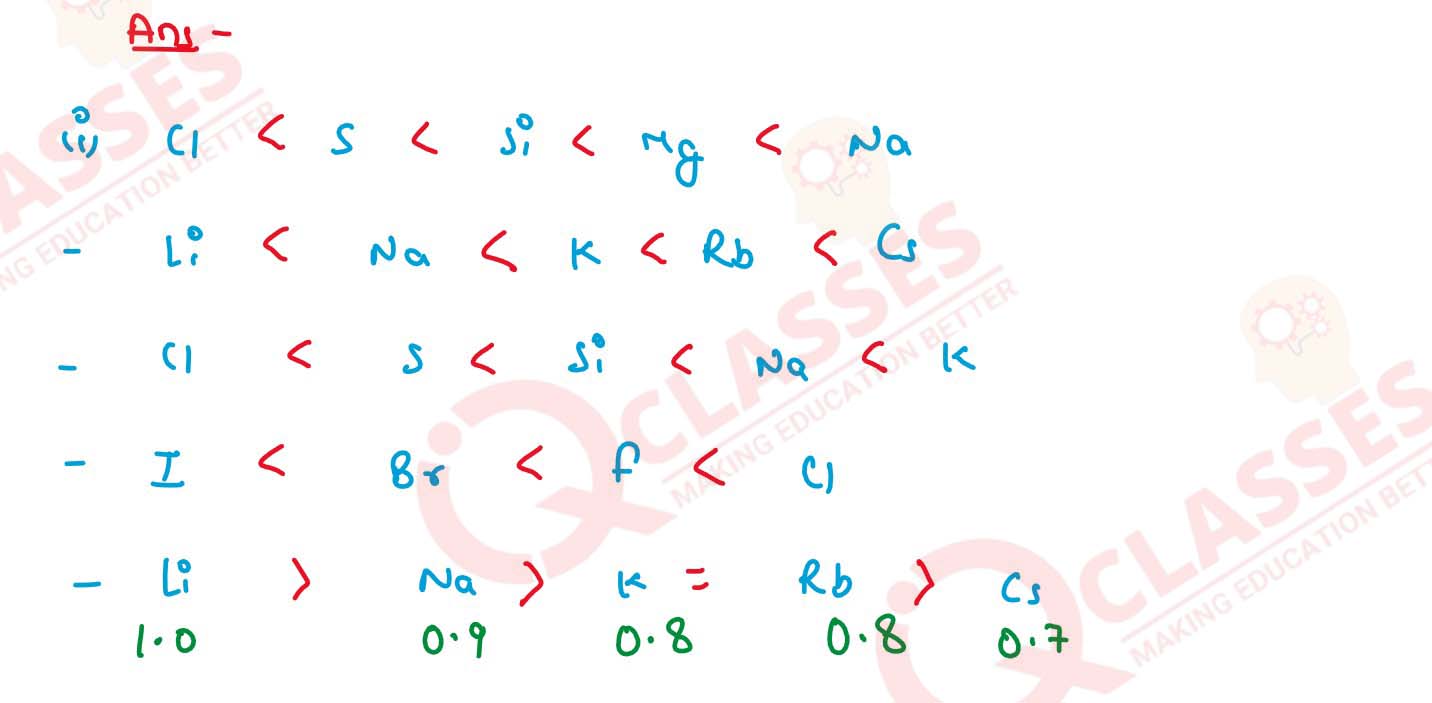
(a) Which of these have Physical and Chemical properties resembling chlorine ?
(b) Which is more electronegative than chlorine ?
Solution

4
First ionisation enthalpy of two clements X and Y are
500 kJ mol-1 and 375 kJ mol-1 respectively. Comment
about their relative position in a group as well as in a
period.
Solution
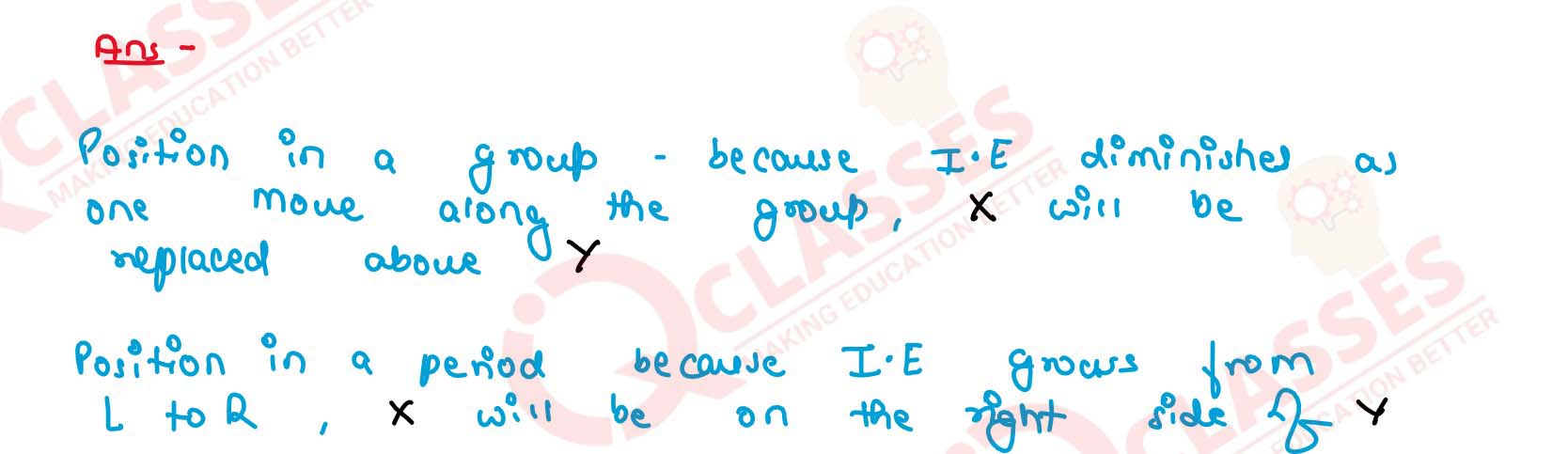
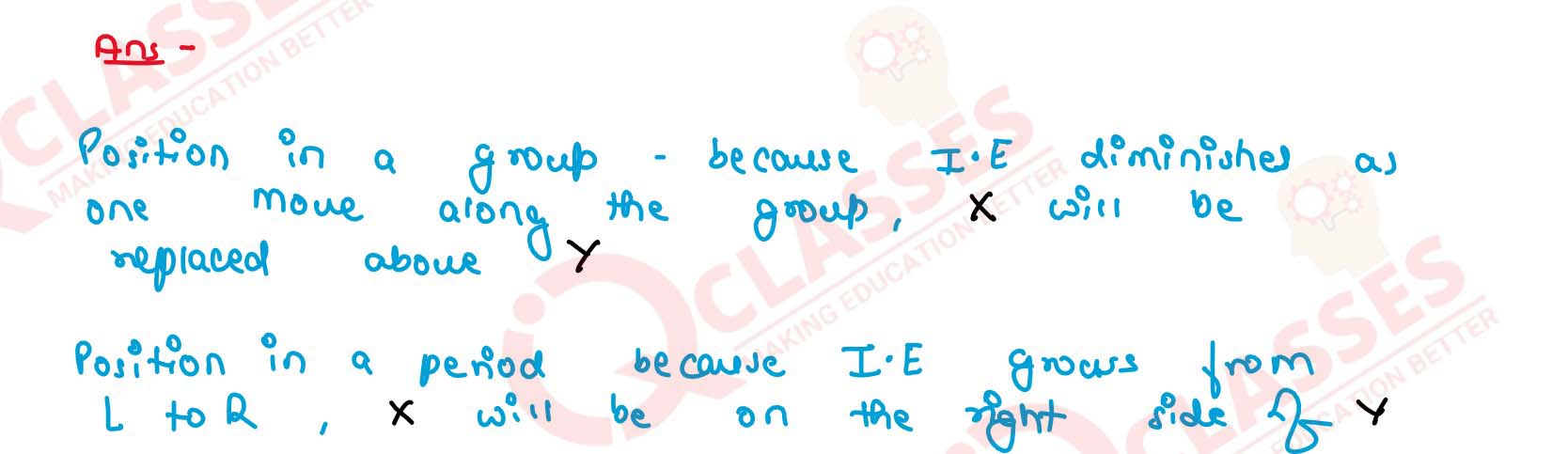
5
Arrange the following in order of increasing radii :
(a) Cl-,Cl (b) Mg2+, Mg, Mg+ (c) N, O, P Solution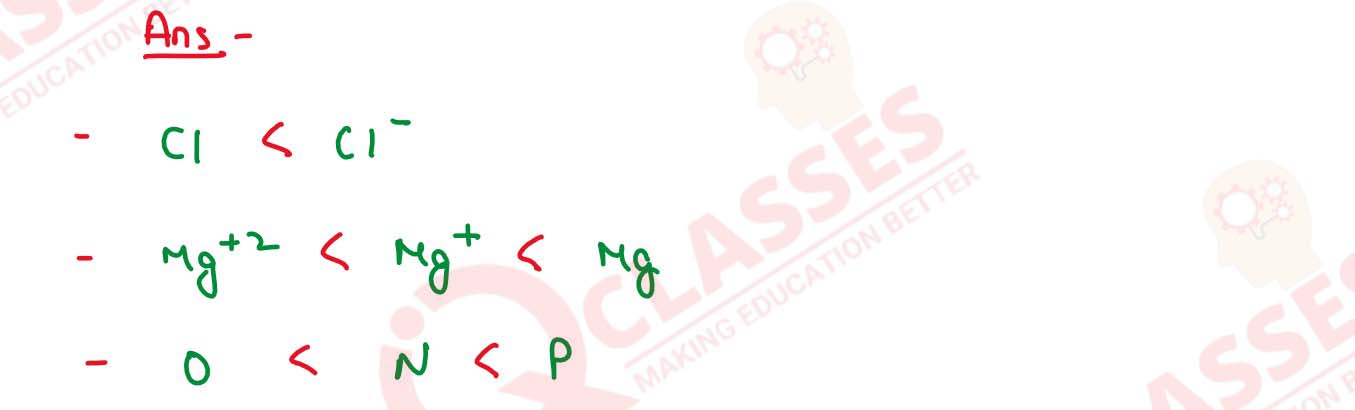
(a) Cl-,Cl (b) Mg2+, Mg, Mg+ (c) N, O, P Solution

6
Which clement from the following has the highest
ionisation energy ?
(a) P, Na, Cl
(b) F, O, Ne
(c) Ne, He, Ar
Explain your choice. Solution
(a) P, Na, Cl
(b) F, O, Ne
(c) Ne, He, Ar
Explain your choice. Solution

7
The electronegativities (according to Pauling) of the
elements in Period 3 of the Periodic Table are as follows
with the elements arranged in alphabetical order:
Arrange the elements in the order in which they occur
in the Periodic Table from left to right.
(The group 1 element first, followed by the group 2 element and so on, up to group 7). Solution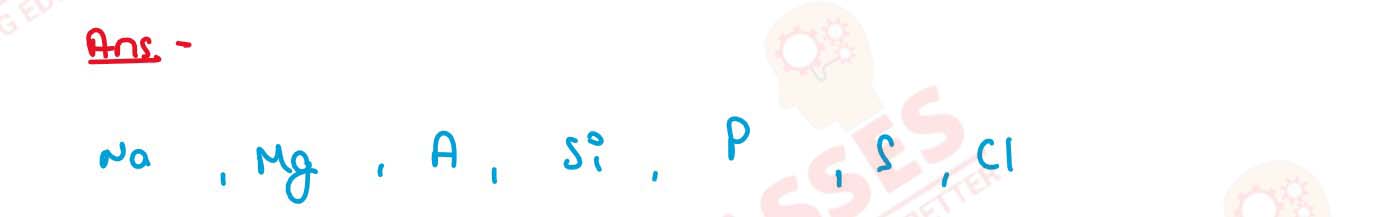
| Al | Cl | Mg | Na | P | S | Si |
| 1.5 | 3.0 | 1.2 | 0.9 | 2.1 | 2.5 | 1.8 |
(The group 1 element first, followed by the group 2 element and so on, up to group 7). Solution
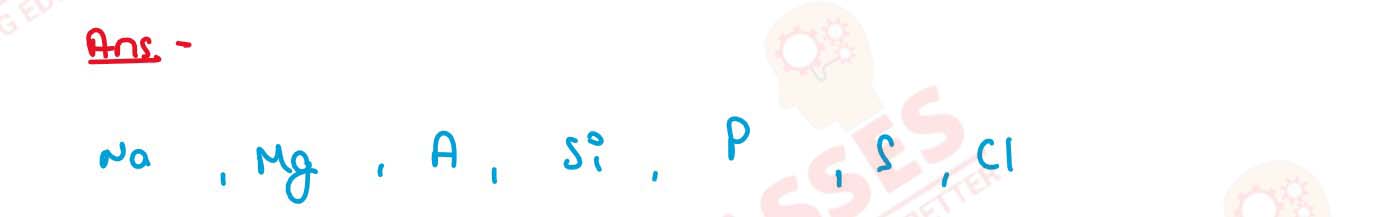
8
Choose the word or phrase from the brackets which
correctly completes each of the following statements :-
(i) The element below sodium in the same group would be expected to have a ......... (lower/higher) electro-negativity than sodium and the element above chlorine would be expected to have 4 ............. (lower/higher) ionization potential than chlorine.
(ii) On moving from left to right in a given period, the number of shells ............. (remains the same/ increases decreases).
(iii) On moving down a group, the number of valence electrons ............. (remains the same/increases/ decreases).
(iv) Metals are good ........... (oxidising agent/ reducing agent) because they are electron ............ (acceptors/donors). Solution
(i) The element below sodium in the same group would be expected to have a ......... (lower/higher) electro-negativity than sodium and the element above chlorine would be expected to have 4 ............. (lower/higher) ionization potential than chlorine.
(ii) On moving from left to right in a given period, the number of shells ............. (remains the same/ increases decreases).
(iii) On moving down a group, the number of valence electrons ............. (remains the same/increases/ decreases).
(iv) Metals are good ........... (oxidising agent/ reducing agent) because they are electron ............ (acceptors/donors). Solution

9
Parts (a) to (e) refer to changes in the properties of
elements on moving from left to right across a period of
the Periodic Table. For each property, choose the correct
answer.
(a) The non-metallic character of the elements :
(i) decreases,
(ii) increases,
(iii) remains the same,
(iv) depends on the period
(b) The electronegativity :
(i) depends on the number of valence electrons,
(ii) remains the same,
(iii) decreases,
(iv) increases.
(c) The ionization potential :
(i) goes up and down
(ii) decreases
(iii) increases
(iv) remains the same
(d) The atomic size :
(i) decreases,
(ii) increases,
(iii) remains the same,
(iv) sometimes increases and sometimes decreases
(e) The electron affinity of the elements 1 to 7:
(i) goes up and then down.
(ii) decreases and then increases,
(iii) increases in groups
(iv) decreases.
Solution
(a) The non-metallic character of the elements :
(i) decreases,
(ii) increases,
(iii) remains the same,
(iv) depends on the period
(b) The electronegativity :
(i) depends on the number of valence electrons,
(ii) remains the same,
(iii) decreases,
(iv) increases.
(c) The ionization potential :
(i) goes up and down
(ii) decreases
(iii) increases
(iv) remains the same
(d) The atomic size :
(i) decreases,
(ii) increases,
(iii) remains the same,
(iv) sometimes increases and sometimes decreases
(e) The electron affinity of the elements 1 to 7:
(i) goes up and then down.
(ii) decreases and then increases,
(iii) increases in groups
(iv) decreases.
Solution

10
The elements of one short period of the Periodic Table are given below in order from left to right :
Li Be B C O F Ne
(a) To which period do these elements belong ?
(b) One element of this period is missing. Which is the missing element and where should it be placed ?
(c) Place the three elements fluorine, beryllium and nitrogen in the order of increasing electronegativity.
(d) Which one of the above elements belongs to the halogen series ?
Solution
Li Be B C O F Ne
(a) To which period do these elements belong ?
(b) One element of this period is missing. Which is the missing element and where should it be placed ?
(c) Place the three elements fluorine, beryllium and nitrogen in the order of increasing electronegativity.
(d) Which one of the above elements belongs to the halogen series ?
Solution

11
With reference to the variation of properties in
the Periodic Table, which of the following is generally
true?
(a) Atomic size increases from left to right across a period.
(b) Ionization potential increases from left to right across a period.
(c) Electron affinity increases going down a group.
(d) Electro-negativity increases going down a group.
Solution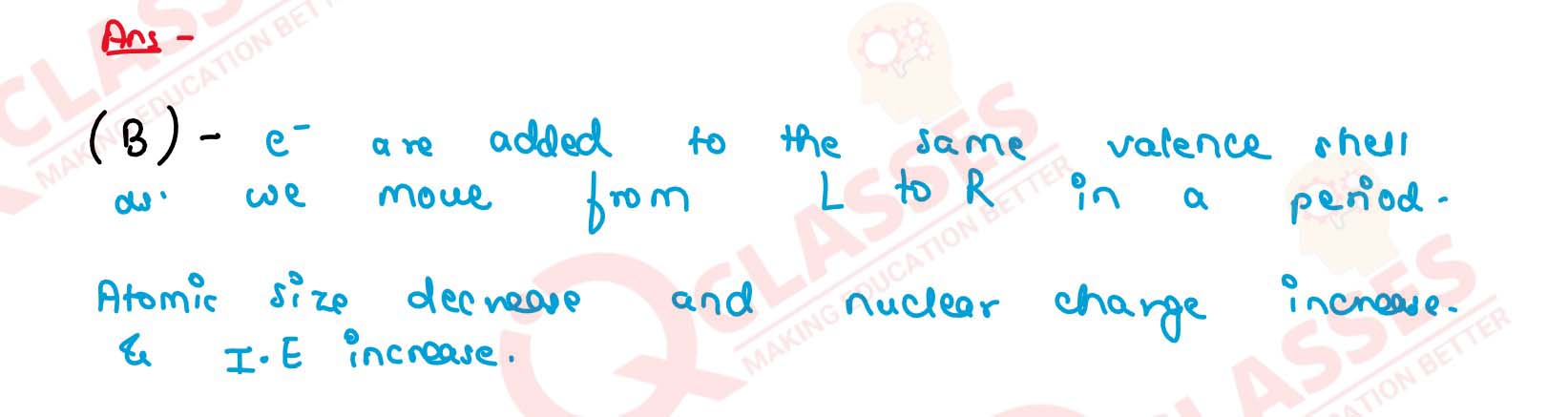
(a) Atomic size increases from left to right across a period.
(b) Ionization potential increases from left to right across a period.
(c) Electron affinity increases going down a group.
(d) Electro-negativity increases going down a group.
Solution
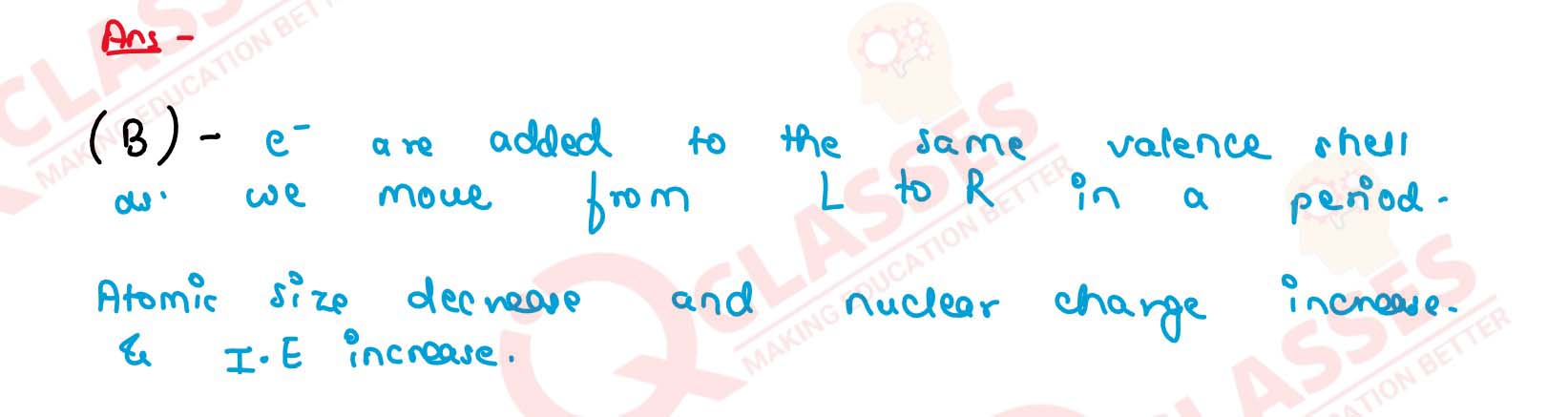
12
Atomic numbers of elements A, B, C, D, E, F are 8, 7, 11.
12, 13 and 9 respectively. State the type of ions they form.
Solution


13
(a) Formula of ion of A is A2+. Element A probably
belongs to ............ group.
(b) In a period, increase in electron affinity increases .............. (oxidation/reduction).
(c) On descending a group, .......... (increase/ decrease) in ionisation potential as well as electron affinity ........... (increases/decreases) oxidising capacity. Solution
(b) In a period, increase in electron affinity increases .............. (oxidation/reduction).
(c) On descending a group, .......... (increase/ decrease) in ionisation potential as well as electron affinity ........... (increases/decreases) oxidising capacity. Solution
