Q1
Why is water not added to concentrated H2SO4 in order
to dilute it ?
solutions
solutions

Q2
Give two balanced reactions of each type to show the
following properties of sulphuric acid :
(a) Acidic nature,
(b) Oxidising agent,
(c) Dehydrating nature,
(d) Non-volatile nature.
solutions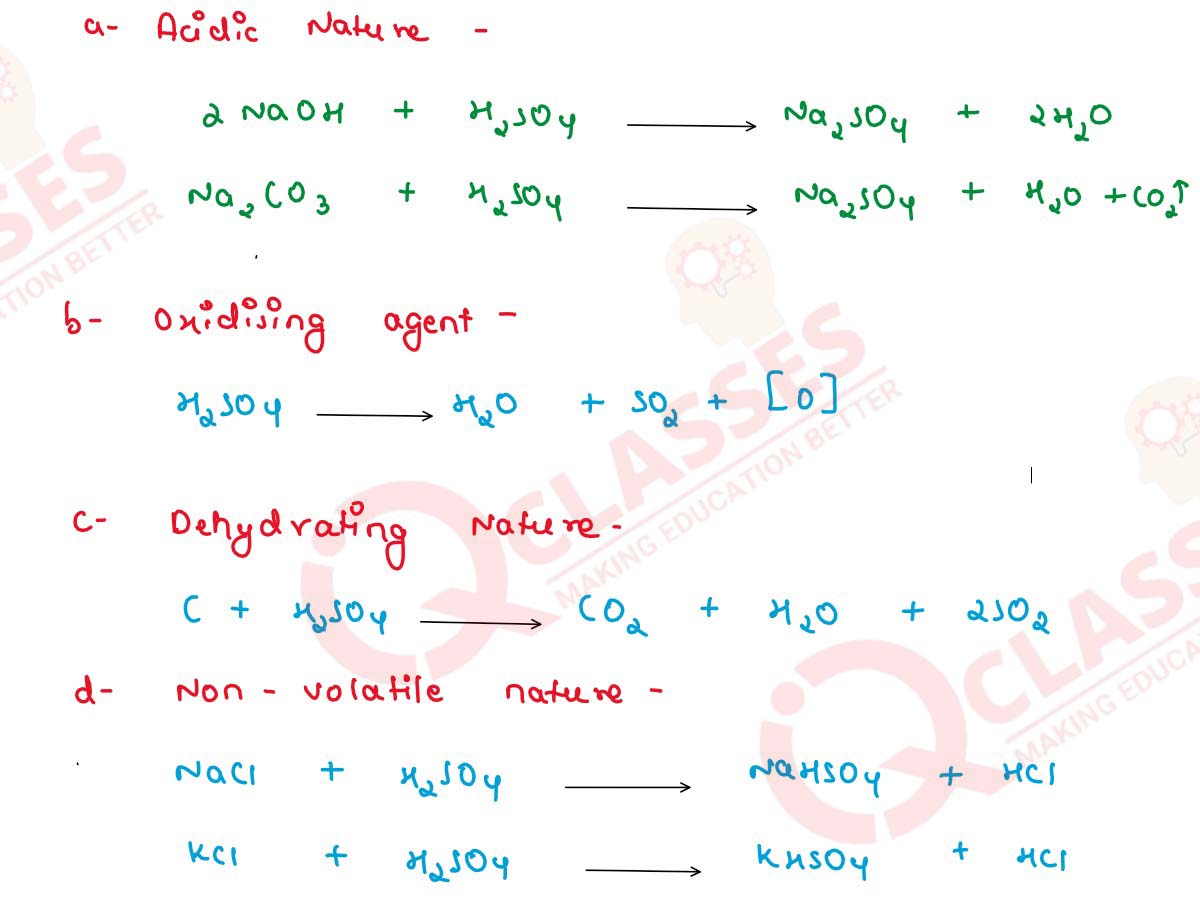
(a) Acidic nature,
(b) Oxidising agent,
(c) Dehydrating nature,
(d) Non-volatile nature.
solutions

Q3
Give a chemical test to distinguish between :
(a) dilute sulphuric acid and dilute hydrochloric acid, (using lead nitrate solution).
(b) dilute sulphuric acid and conc. sulphuric acid.
solutions
(a) dilute sulphuric acid and dilute hydrochloric acid, (using lead nitrate solution).
(b) dilute sulphuric acid and conc. sulphuric acid.
solutions

Q4
Write balanced chemical equations :
when hot and concentrated sulphuric acid reacts with the following :
(a) Sulphur,
(b) NaOH,
(c) Sugar,
(d) Carbon,
(e) Copper.
solutions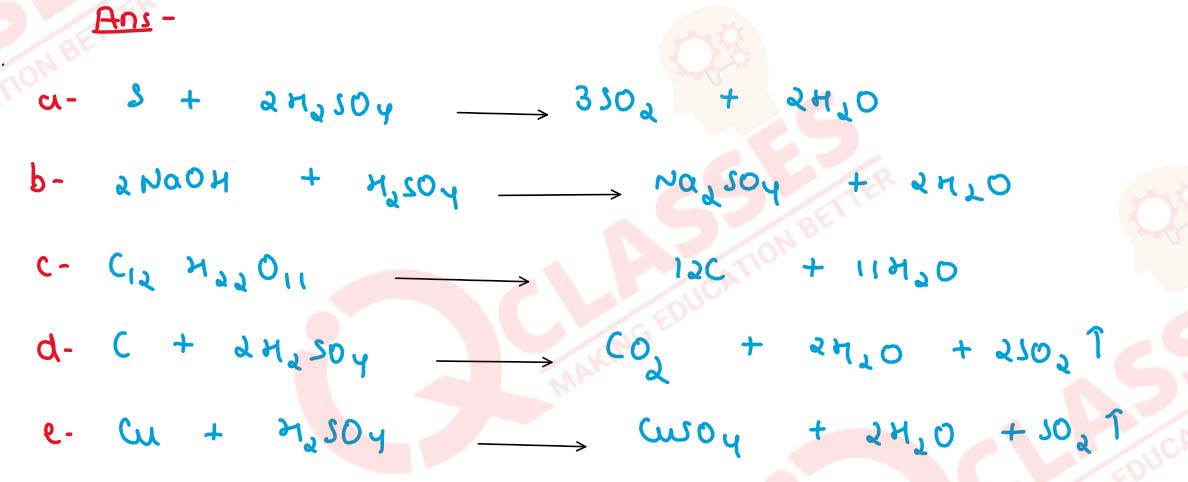
when hot and concentrated sulphuric acid reacts with the following :
(a) Sulphur,
(b) NaOH,
(c) Sugar,
(d) Carbon,
(e) Copper.
solutions

Q5
Why is:
(a) concentrated sulphuric acid kept in air tight bottles ?
(b) H2SO4 is not used as a drying agent for H2S ?
(c) sulphuric acid used in the preparation of HCl and HNO3 ? Give equations in both cases.
solutions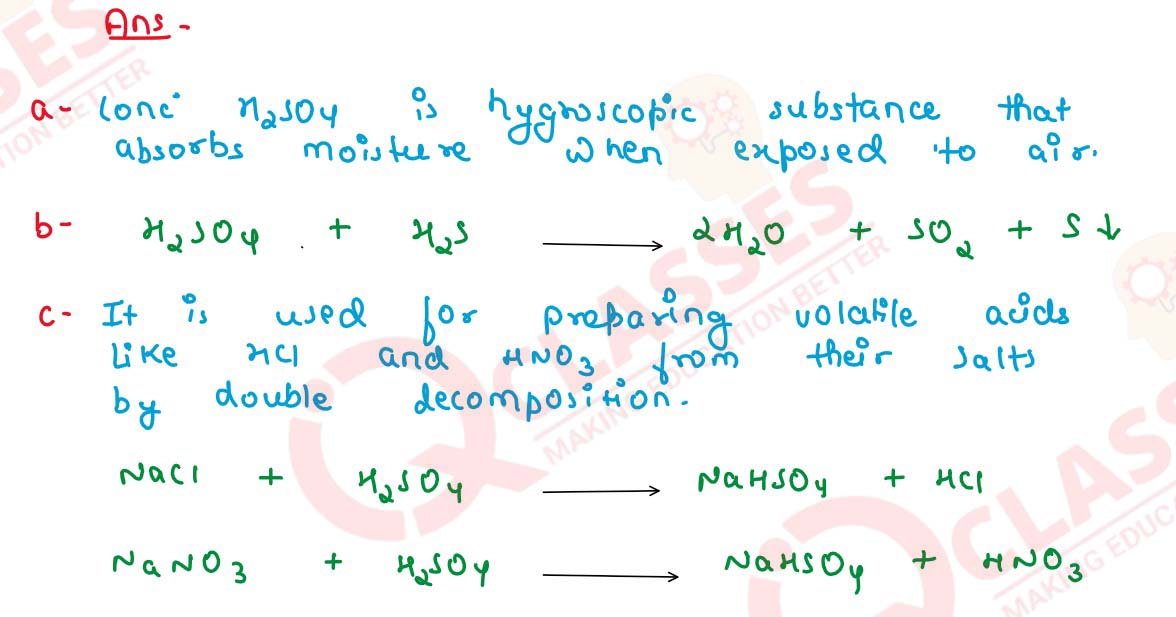
(a) concentrated sulphuric acid kept in air tight bottles ?
(b) H2SO4 is not used as a drying agent for H2S ?
(c) sulphuric acid used in the preparation of HCl and HNO3 ? Give equations in both cases.
solutions

Q6
What property of conc. H2SO4 is made use of in each of
the following cases? Give an equation for the reaction
in each case :
(a) in the production of HCl gas when it reacts with a chloride,
(b) in the preparation of CO from HCOOH,
(c) as a source of hydrogen by diluting it and adding a strip of magnesium,
(d) in the preparation of sulphur dioxide by warming a mixture of conc. sulphuric acid and copper-turnings,
(e) hydrogen chloride gas is passed through concentrated sulphuric acid.
(f) its reaction with (i) Ethanol (ii) Carbon.
solutions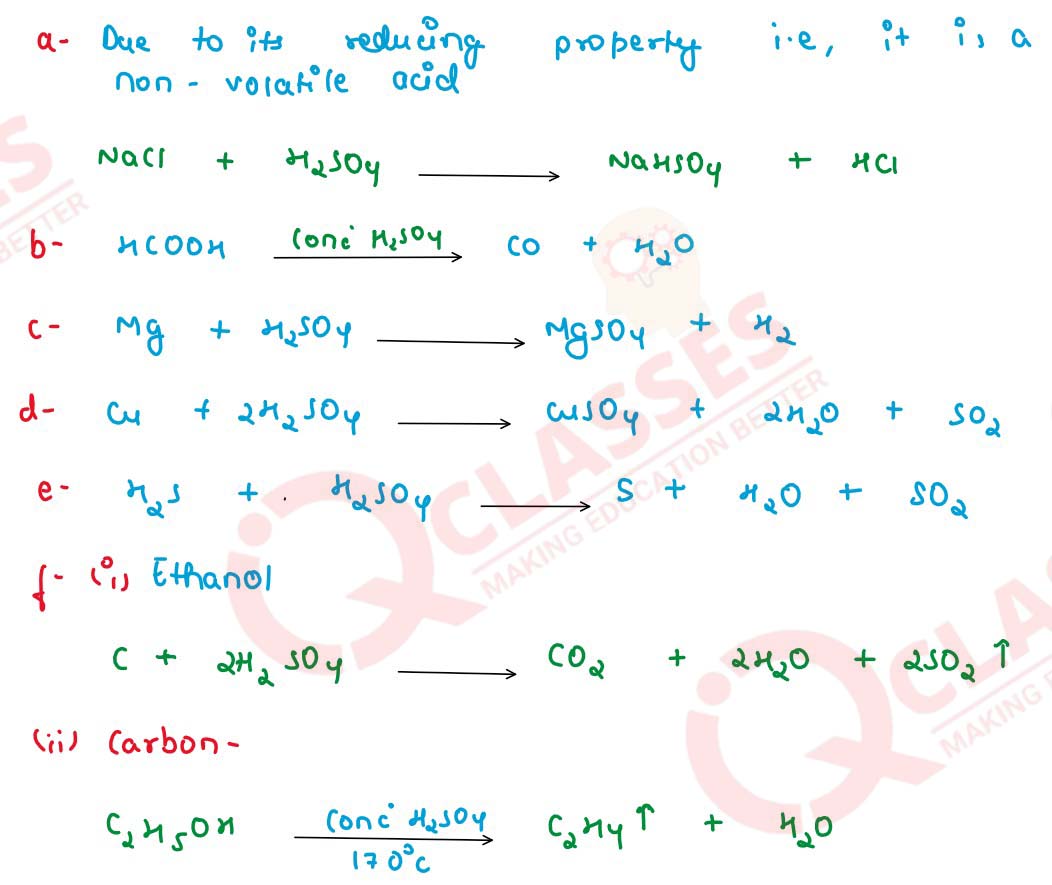
(a) in the production of HCl gas when it reacts with a chloride,
(b) in the preparation of CO from HCOOH,
(c) as a source of hydrogen by diluting it and adding a strip of magnesium,
(d) in the preparation of sulphur dioxide by warming a mixture of conc. sulphuric acid and copper-turnings,
(e) hydrogen chloride gas is passed through concentrated sulphuric acid.
(f) its reaction with (i) Ethanol (ii) Carbon.
solutions

Q7
What is the name given to the salts of :
(a) sulphurous acid, (b) sulphuric acid ?
solutions
(a) sulphurous acid, (b) sulphuric acid ?
solutions

Q8
Give reasons for the following :
(a) Sulphuric acid forms two types of salts with NaOH,
(b) A piece of wood becomes black when concentrated sulphuric acid is poured on it,
(c) Brisk effervescence is seen when oil of vitriol is added to sodium carbonate.
solutions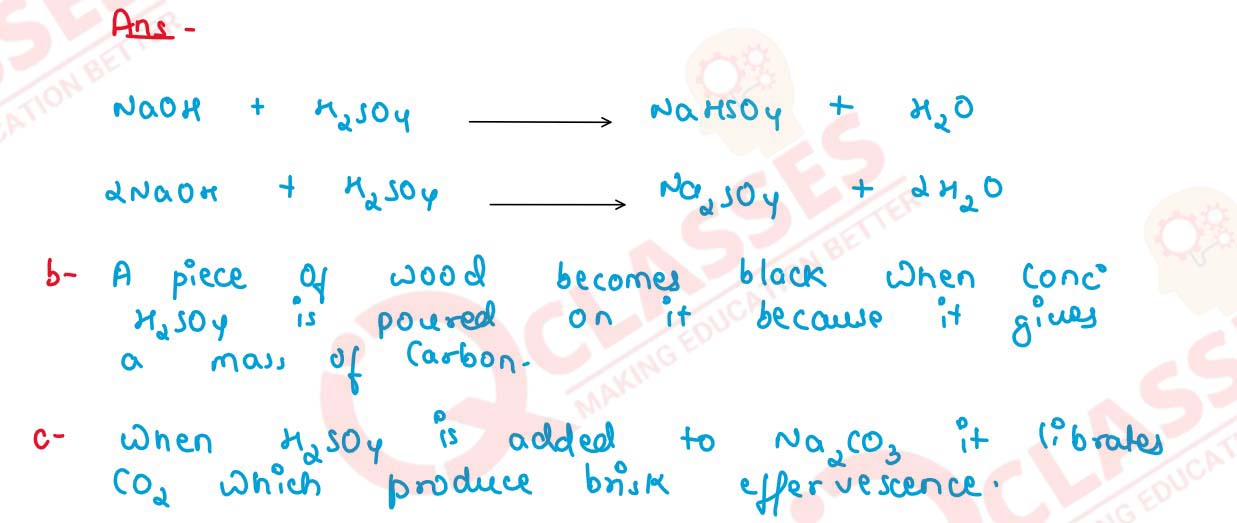
(a) Sulphuric acid forms two types of salts with NaOH,
(b) A piece of wood becomes black when concentrated sulphuric acid is poured on it,
(c) Brisk effervescence is seen when oil of vitriol is added to sodium carbonate.
solutions

Q9
Copy and complete the following table :
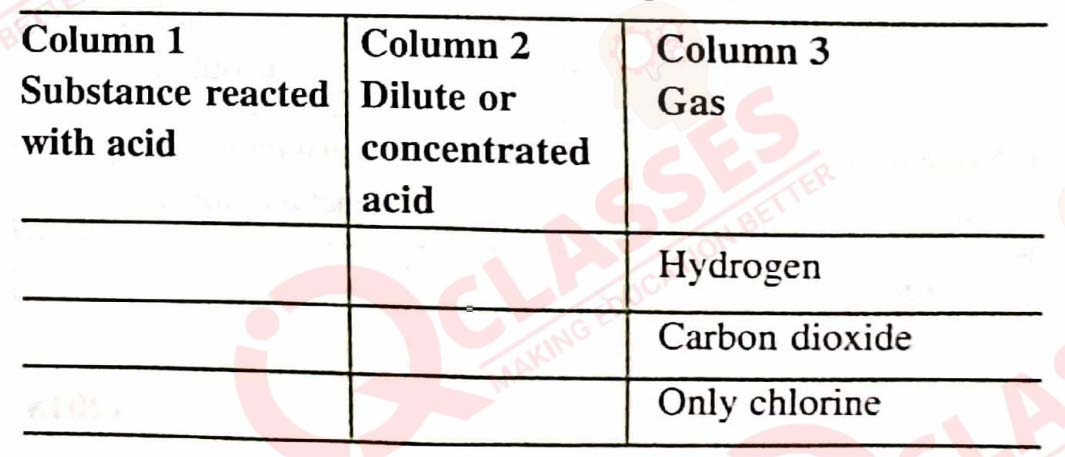
solutions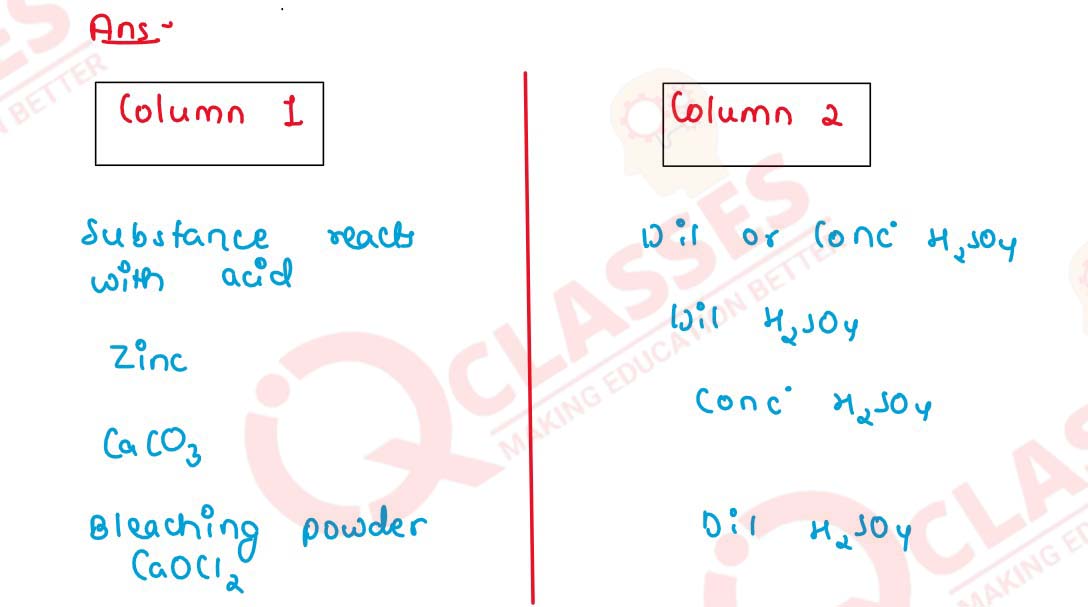

solutions

Q10
Following are the typical properties of dilute acid.
Complete them by inserting suitable words :
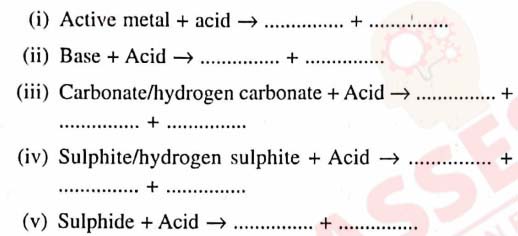
solutions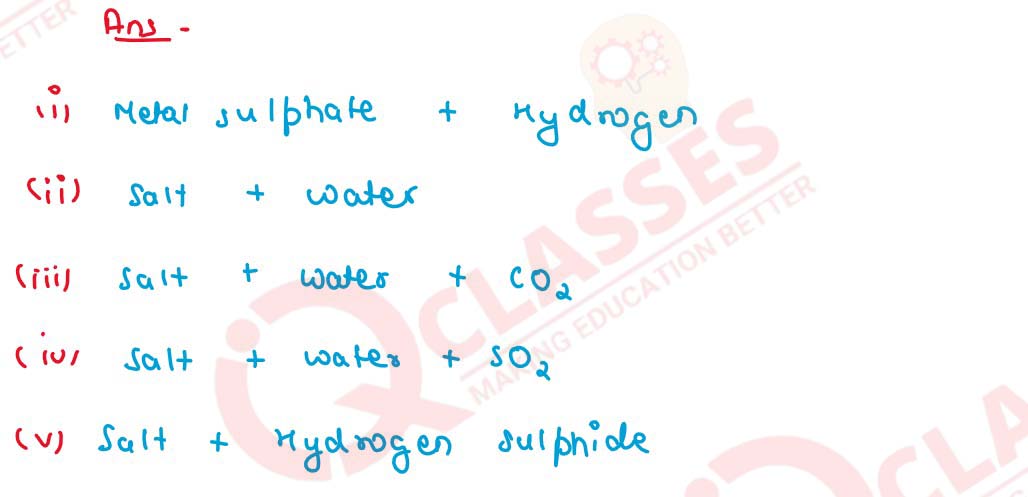

solutions

Q11
(a) Which property of sulphuric acid accounts for its
use as a dehydrating agent ?
(b) Concentrated sulphuric acid is both an oxidizing agent and a non-volatile acid. Write one equation each to illustrate the above mentioned properties of sulphuric acid.
solutions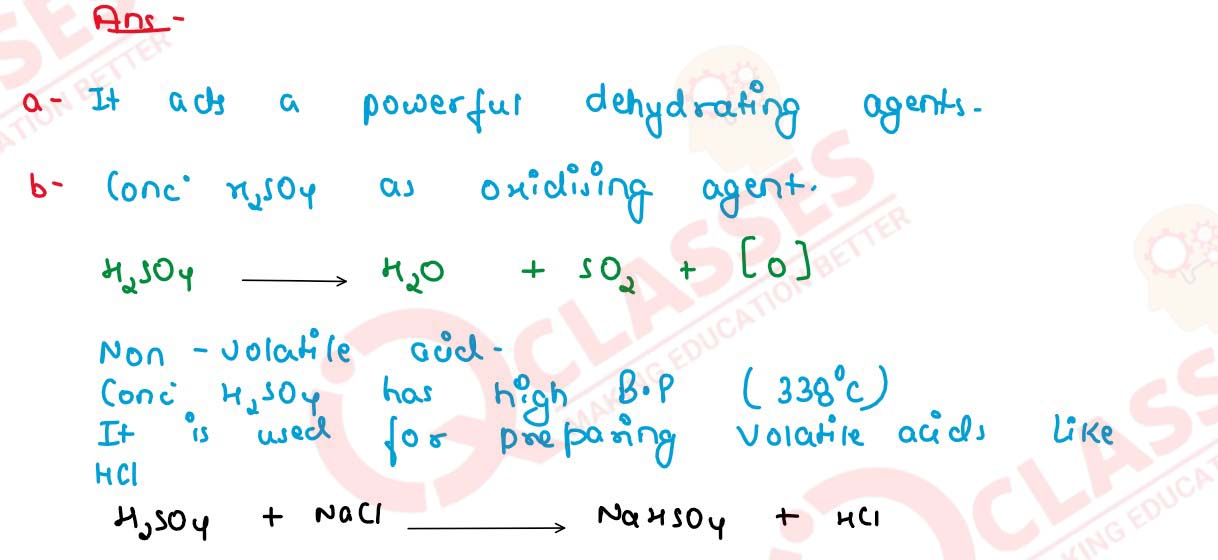
(b) Concentrated sulphuric acid is both an oxidizing agent and a non-volatile acid. Write one equation each to illustrate the above mentioned properties of sulphuric acid.
solutions

Q12
Some properties of Sulphuric acid are listed below.
Choose the property A, B, C or D which is responsible
for the reactions (i) to (v). Some properties may be
repeated :
A. Typical acid
B. Dehydrating agent
C. Non-volatile acid
D. Oxidizing agent
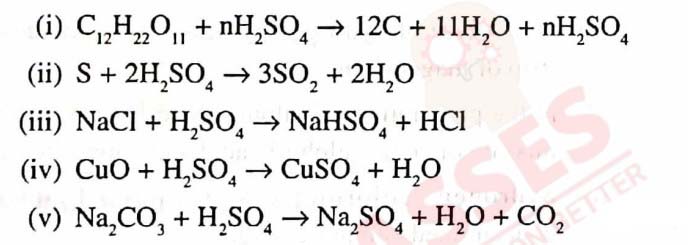
solutions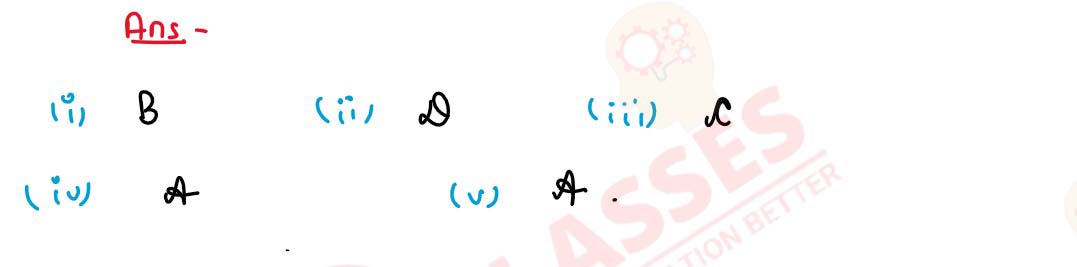
A. Typical acid
B. Dehydrating agent
C. Non-volatile acid
D. Oxidizing agent
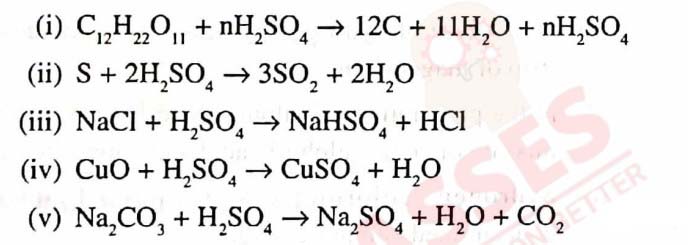
solutions

Q13
(a) Name the acid formed when sulphur dioxide
dissolves in water.
(b) Name the gas released when sodium carbonate is added to a solution of sulphur dioxide.
solutions
(b) Name the gas released when sodium carbonate is added to a solution of sulphur dioxide.
solutions

Q14 [2008]
(a) Dilute sulphuric acid will produce a white precipitate when added to a solution of :
(i) Copper nitrate
(ii) Zinc nitrate
(iii) Lead nitrate
(iv) Sodium nitrate
(b) Identify the following substance : Liquid E can be dehydrated to produce ethene.
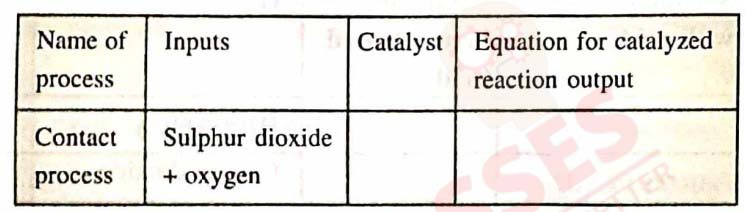
(c) Copy and complete the following table relating to an important industrial process and its final output.
(d) Making use only of substances given : dil. sulphuric acid, sodium carbonate, zinc, sodium sulphite, lead, calcium carbonate :
Give equations for the reactions by which you could obtain :
(i) hydrogen
(ii) sulphur dioxide
(iii) carbon dioxide
(iv) zinc carbonate [2 steps]
(e) What property of conc. H2SO4 :
(i) is used in the action when sugar turns black in its presence.
(ii) allows it to be used in the preparation of HCl and HNO3 acids.
(f) Write the equations for :
(i) dil. H2SO4 and barium chloride
(ii) dil. H2SO4 and sodium sulphide
solutions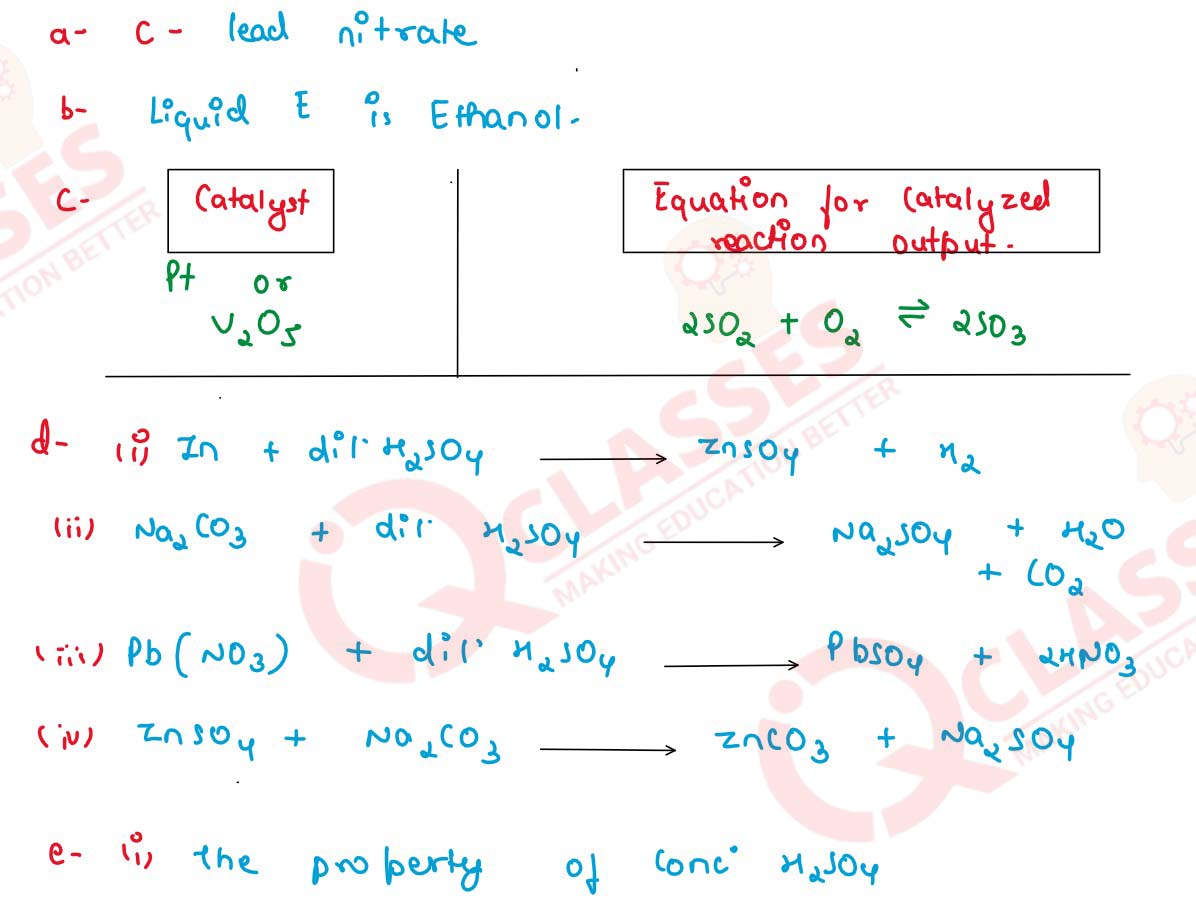
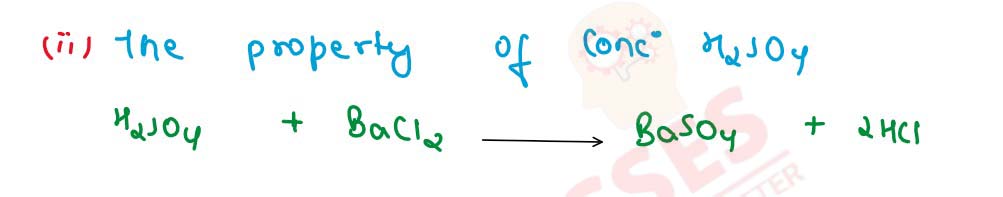
(a) Dilute sulphuric acid will produce a white precipitate when added to a solution of :
(i) Copper nitrate
(ii) Zinc nitrate
(iii) Lead nitrate
(iv) Sodium nitrate
(b) Identify the following substance : Liquid E can be dehydrated to produce ethene.
(c) Copy and complete the following table relating to an important industrial process and its final output.

(d) Making use only of substances given : dil. sulphuric acid, sodium carbonate, zinc, sodium sulphite, lead, calcium carbonate :
Give equations for the reactions by which you could obtain :
(i) hydrogen
(ii) sulphur dioxide
(iii) carbon dioxide
(iv) zinc carbonate [2 steps]
(e) What property of conc. H2SO4 :
(i) is used in the action when sugar turns black in its presence.
(ii) allows it to be used in the preparation of HCl and HNO3 acids.
(f) Write the equations for :
(i) dil. H2SO4 and barium chloride
(ii) dil. H2SO4 and sodium sulphide
solutions

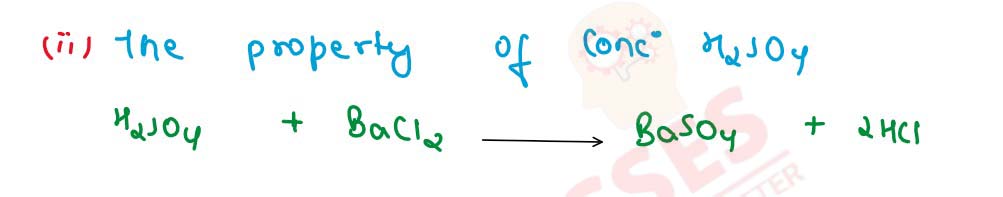
Q15 [2009]
(a) Name the gas evolved [formula is not acceptable]. The gas that can be oxidised to sulphur.
solutions
(a) Name the gas evolved [formula is not acceptable]. The gas that can be oxidised to sulphur.
solutions

Q16 [2010]
(a) Give the equation for :
(i) Heat on sulphur with conc. sulphuric acid.
(ii) Reaction of — sugar with conc. sulphuric acid.
(b) Give a balanced equation for the conversion of zinc oxide to zinc sulphate.
(c) Select the correct answer from A, B, C.
A. Sodium hydroxide solution
B. A weak acid
C. Dilute sulphuric acid.
The solution which liberates sulphur dioxide gas, from sodium sulphite.
solutions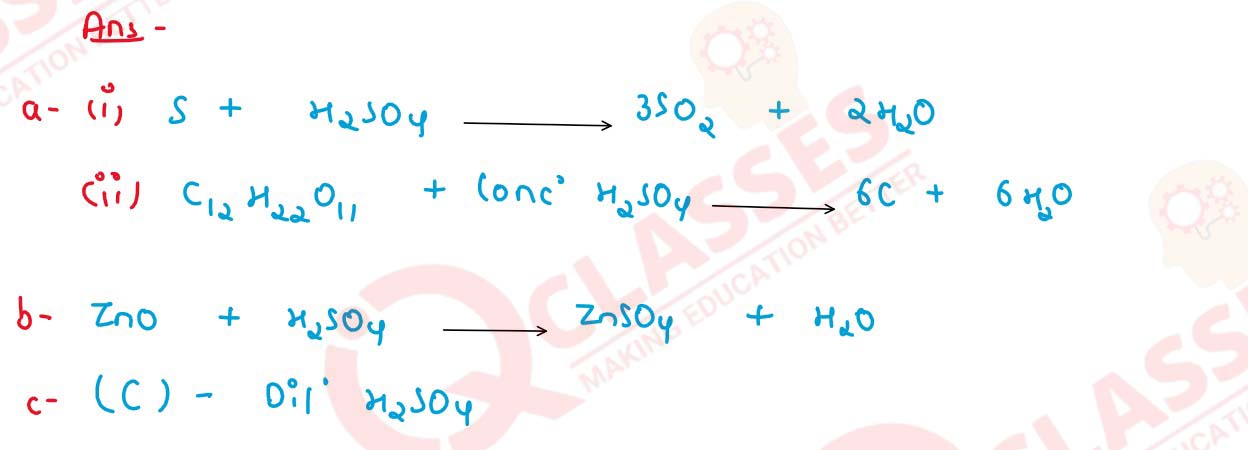
(a) Give the equation for :
(i) Heat on sulphur with conc. sulphuric acid.
(ii) Reaction of — sugar with conc. sulphuric acid.
(b) Give a balanced equation for the conversion of zinc oxide to zinc sulphate.
(c) Select the correct answer from A, B, C.
A. Sodium hydroxide solution
B. A weak acid
C. Dilute sulphuric acid.
The solution which liberates sulphur dioxide gas, from sodium sulphite.
solutions
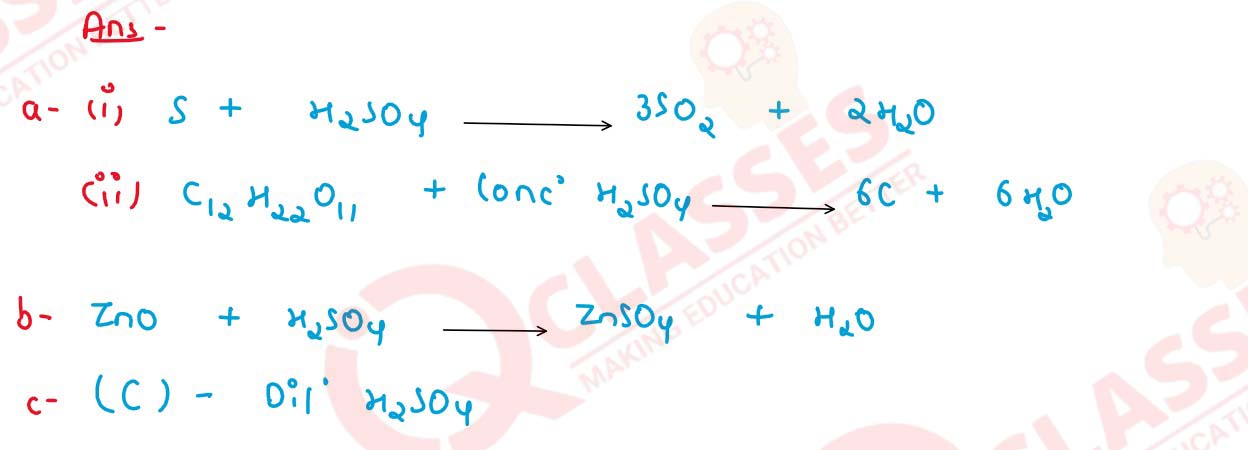
Q17 [2011]
(a) State your observation when — Sugar crystals are added to a hard glass test tube containing conc. sulphuric acid.
(b) Choose the correct answer from the choices — The gas evolved when dil. sulphuric acid reacts with iron sulphide.
(i) Hydrogen sulphide
(ii) Sulphur dioxide
(iii) Sulphur trioxide
(iv) Vapour of sulphuric acid
(c) Give a balanced equation for :
(i) Dilute sulphuric acid when poured over sodium sulphite.
(ii) Manufacture of sulphuric acid by the contact process.
(d) State the property of sulphuric acid shown by the reaction of conc. sulphuric acid when heated with
(i) potassium nitrate
(ii) carbon
(iii) ethanol
solutions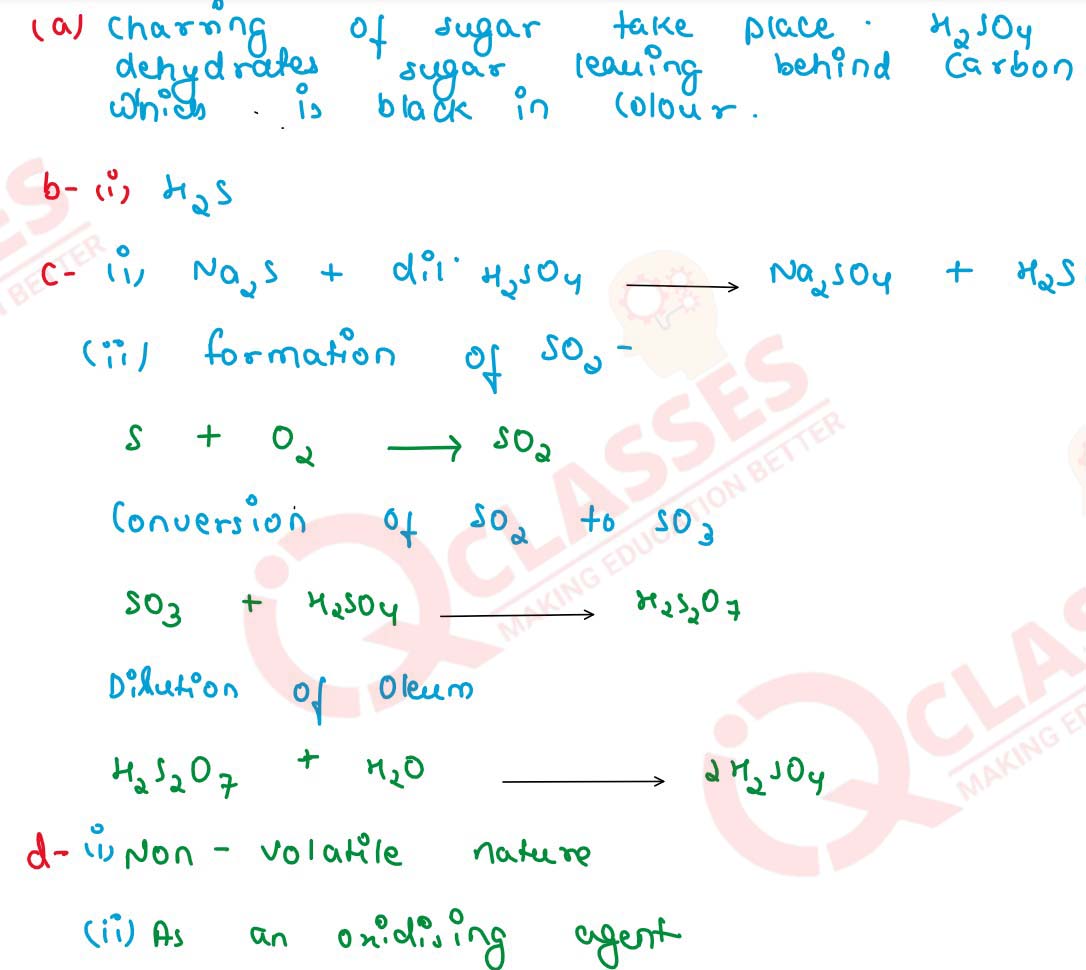
(a) State your observation when — Sugar crystals are added to a hard glass test tube containing conc. sulphuric acid.
(b) Choose the correct answer from the choices — The gas evolved when dil. sulphuric acid reacts with iron sulphide.
(i) Hydrogen sulphide
(ii) Sulphur dioxide
(iii) Sulphur trioxide
(iv) Vapour of sulphuric acid
(c) Give a balanced equation for :
(i) Dilute sulphuric acid when poured over sodium sulphite.
(ii) Manufacture of sulphuric acid by the contact process.
(d) State the property of sulphuric acid shown by the reaction of conc. sulphuric acid when heated with
(i) potassium nitrate
(ii) carbon
(iii) ethanol
solutions

Q18 [2012]
(a) Name — The gas produced on reaction of dilute sulphuric acid with a metallic sulphide.
(b) Some properties of sulphuric acid are listed below. Choose the role played by sulphuric acid as A, B, C or D which is responsible for the reactions (i) to (v).
Some role/s may be repeated.
(A) Dilute acid
(C) Non-volatile acid
(B) Dehydrating agent
(D) Oxidising agent
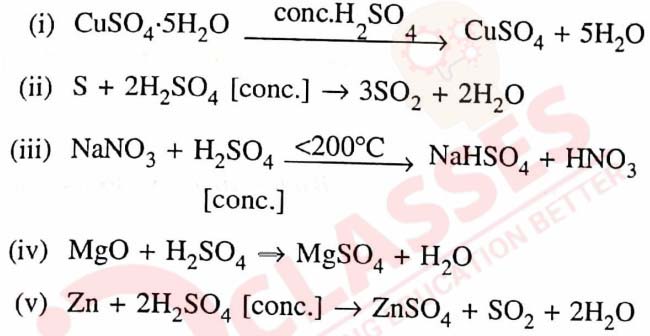
(c) Give balanced equation for the reaction : Zinc sulphide and dilute sulphuric acid.
solutions
(a) Name — The gas produced on reaction of dilute sulphuric acid with a metallic sulphide.
(b) Some properties of sulphuric acid are listed below. Choose the role played by sulphuric acid as A, B, C or D which is responsible for the reactions (i) to (v).
Some role/s may be repeated.
(A) Dilute acid
(C) Non-volatile acid
(B) Dehydrating agent
(D) Oxidising agent
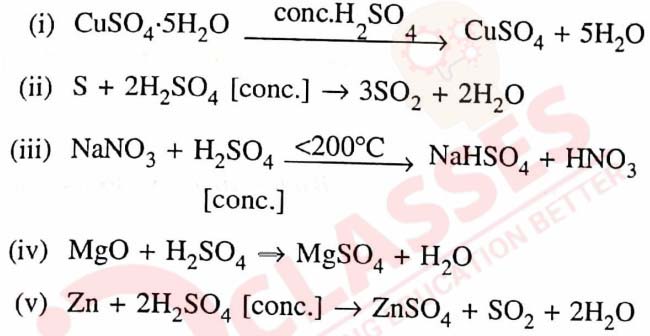
(c) Give balanced equation for the reaction : Zinc sulphide and dilute sulphuric acid.
solutions

Q19 [2013]
(a) State one appropriate observation when : Conc. H2SO4 is added to a crystal of hydrated copper sulphate.
(b) In the given equation — S + 2H2SO4 —> 3SO2 + 2H2O:
Identify the role played by conc. H2SO4.
(i) Non-volatile acid
(ii) Oxidising agent
(iii) Dehydrating agent
(iv) None of the above
(c) Give a balanced equation for : Dehydration of concentrated sulphuric acid with sugar crystals.
(d) Identify the substance underlined ; A dilute mineral acid which forms a white precipitate when treated with barium chloride solution.
solutions
(a) State one appropriate observation when : Conc. H2SO4 is added to a crystal of hydrated copper sulphate.
(b) In the given equation — S + 2H2SO4 —> 3SO2 + 2H2O:
Identify the role played by conc. H2SO4.
(i) Non-volatile acid
(ii) Oxidising agent
(iii) Dehydrating agent
(iv) None of the above
(c) Give a balanced equation for : Dehydration of concentrated sulphuric acid with sugar crystals.
(d) Identify the substance underlined ; A dilute mineral acid which forms a white precipitate when treated with barium chloride solution.
solutions

Q20 [2014]
(a) Write balanced equations for the following : Action of concentrated sulphuric acid on carbon.
(b) Distinguish between the following pairs of compounds using the test given within brackets. Dilute sulphuric acid and dilute hydrochloric acid [using barium chloride solution].
(c) State the conditions required for the following reactions to take place :
The conversion of sulphur dioxide to sulphur trioxide. (d) Give one equation each to show the following properties of sulphuric acid :
(i) Dehydrating property
(ii) Acidic nature
(iii) As a non-volatile acid
solutions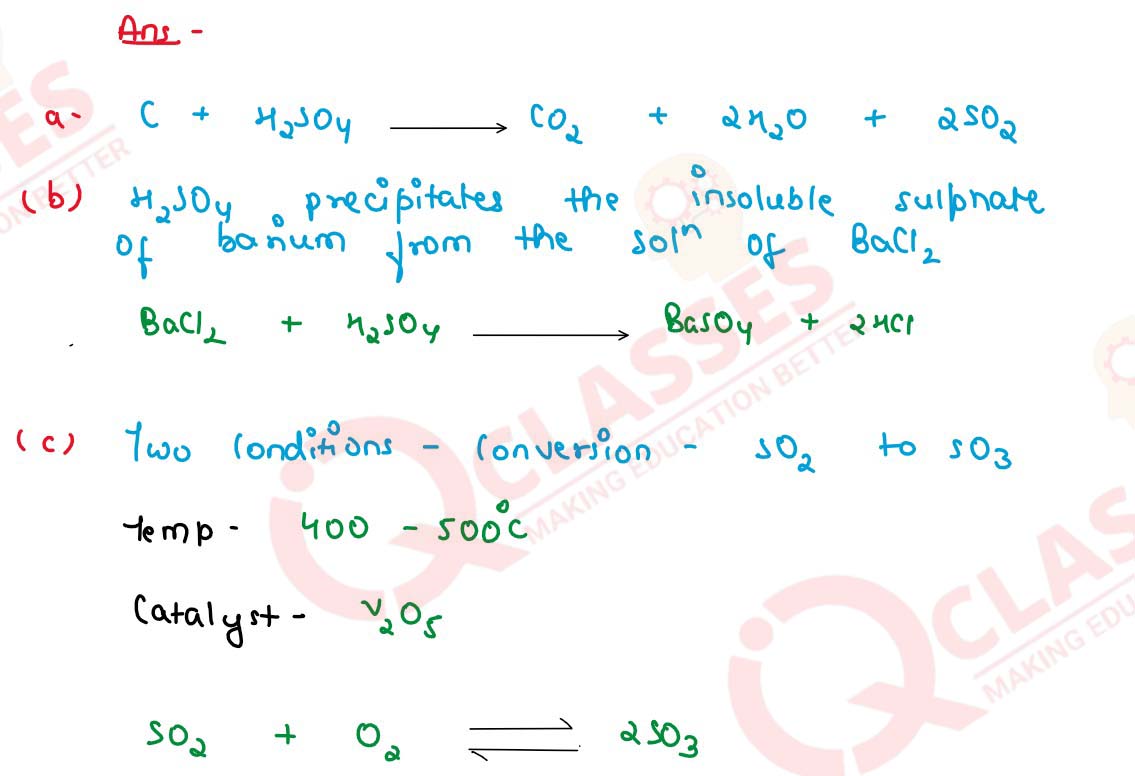

(a) Write balanced equations for the following : Action of concentrated sulphuric acid on carbon.
(b) Distinguish between the following pairs of compounds using the test given within brackets. Dilute sulphuric acid and dilute hydrochloric acid [using barium chloride solution].
(c) State the conditions required for the following reactions to take place :
The conversion of sulphur dioxide to sulphur trioxide. (d) Give one equation each to show the following properties of sulphuric acid :
(i) Dehydrating property
(ii) Acidic nature
(iii) As a non-volatile acid
solutions


Q21 [2015]
(a) In the manufacture of sulphuric acid by contact process give the equations for the conversion of sulphur trioxide to sulphuric acid.
(b) Give equations for the action of sulphuric acid on
(i) Potassium hydrogen carbonate
(ii) Sulphur
(c) Identify the acid in each case
(i) The acid which produces sugar charcoal from sugar.
(ii) The acid on mixing with lead nitrate solution produces white ppt. which is insoluble even on heating.
solutions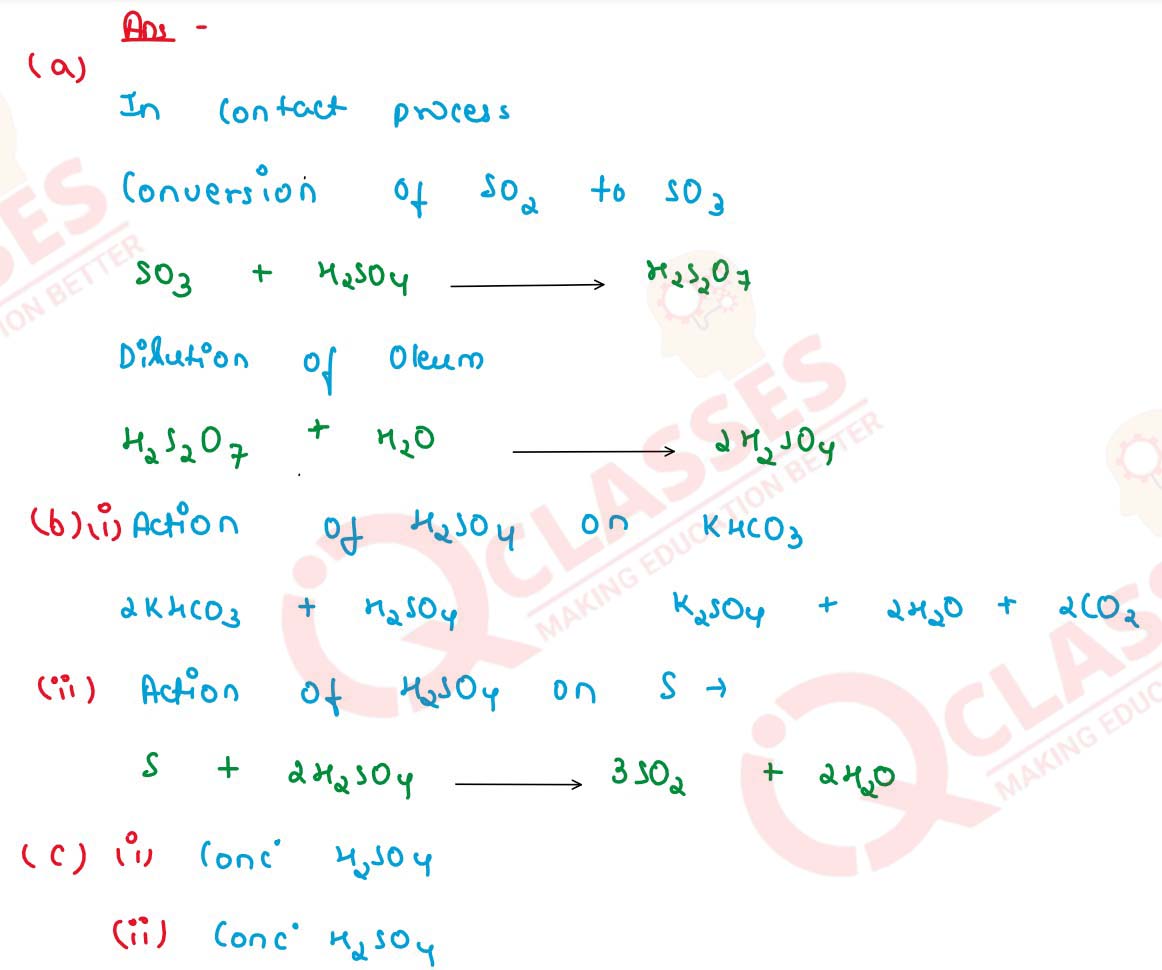
(a) In the manufacture of sulphuric acid by contact process give the equations for the conversion of sulphur trioxide to sulphuric acid.
(b) Give equations for the action of sulphuric acid on
(i) Potassium hydrogen carbonate
(ii) Sulphur
(c) Identify the acid in each case
(i) The acid which produces sugar charcoal from sugar.
(ii) The acid on mixing with lead nitrate solution produces white ppt. which is insoluble even on heating.
solutions
