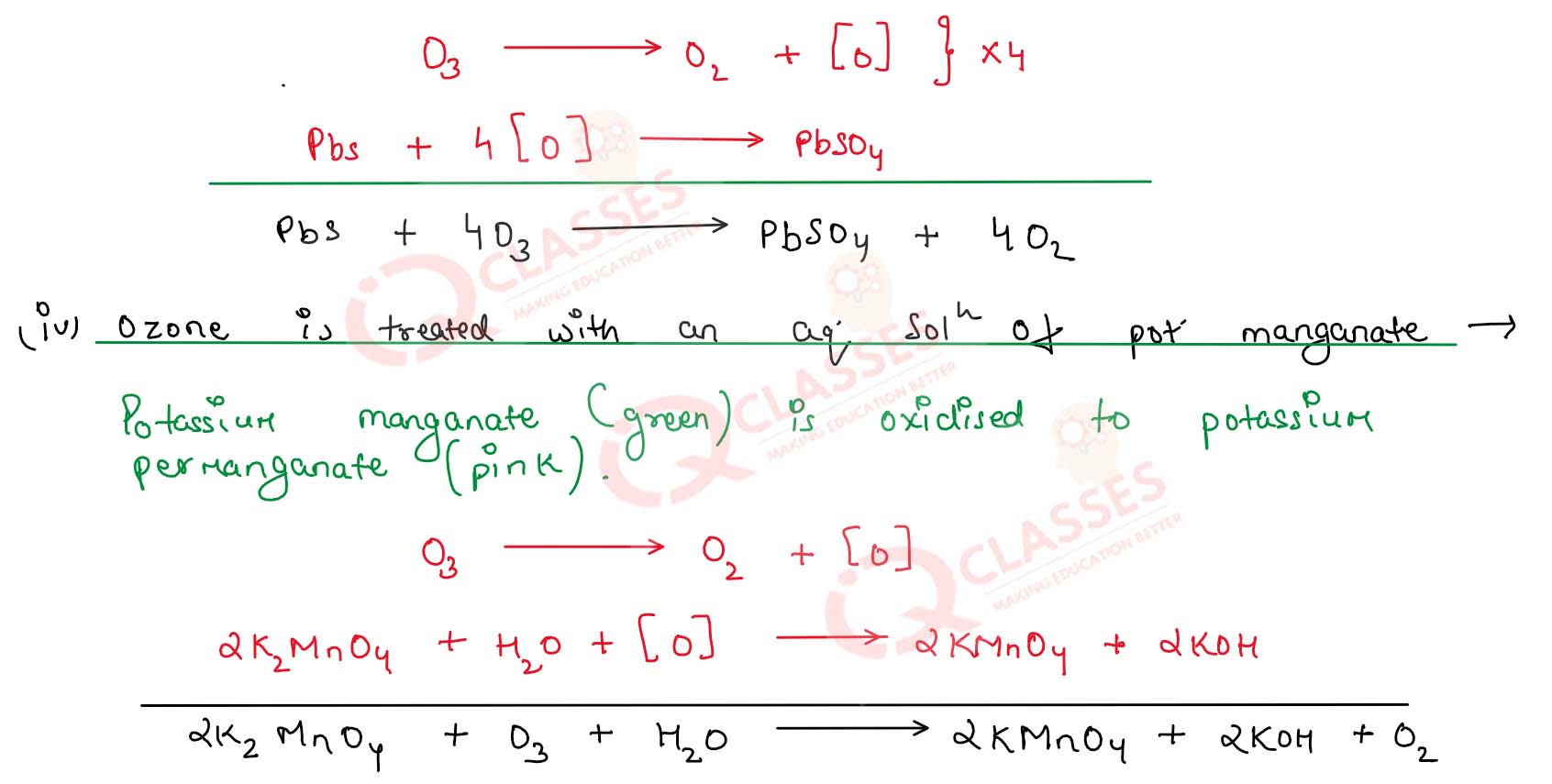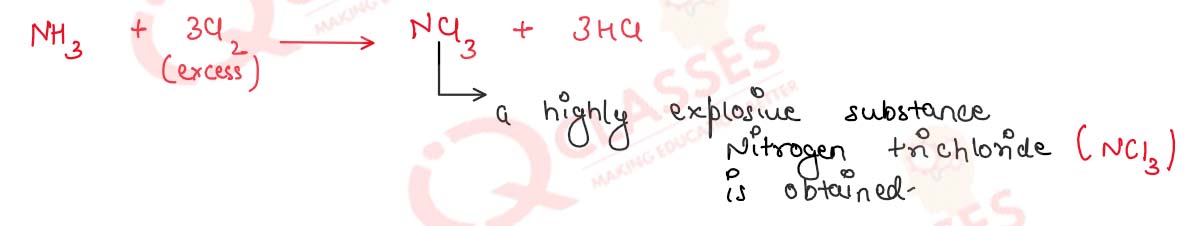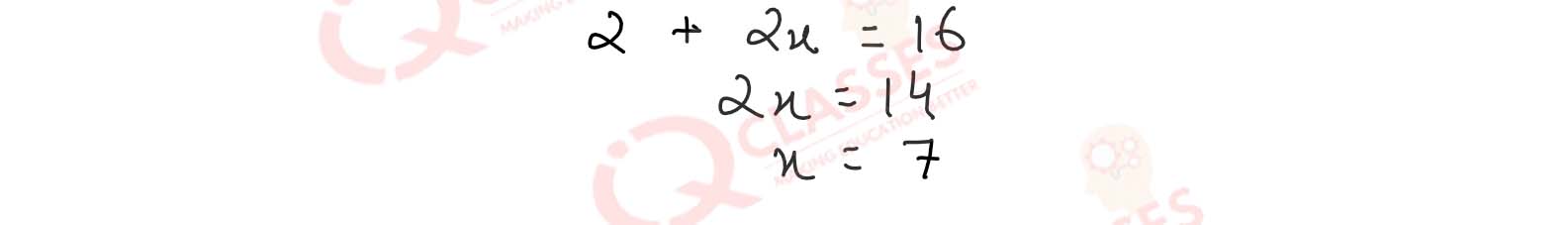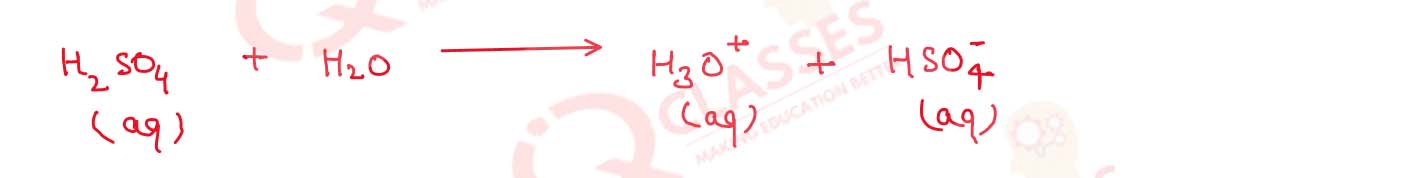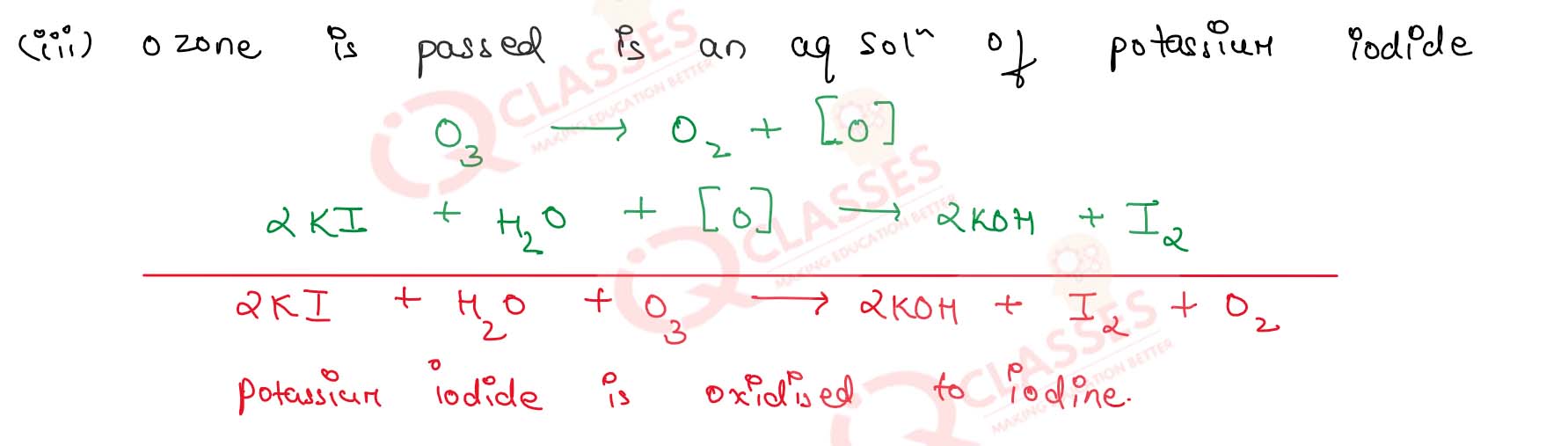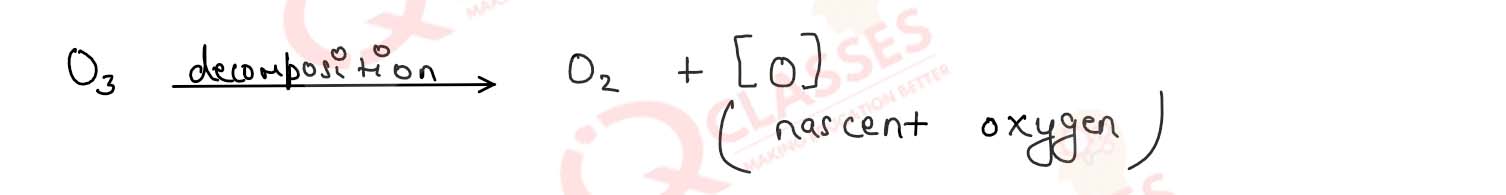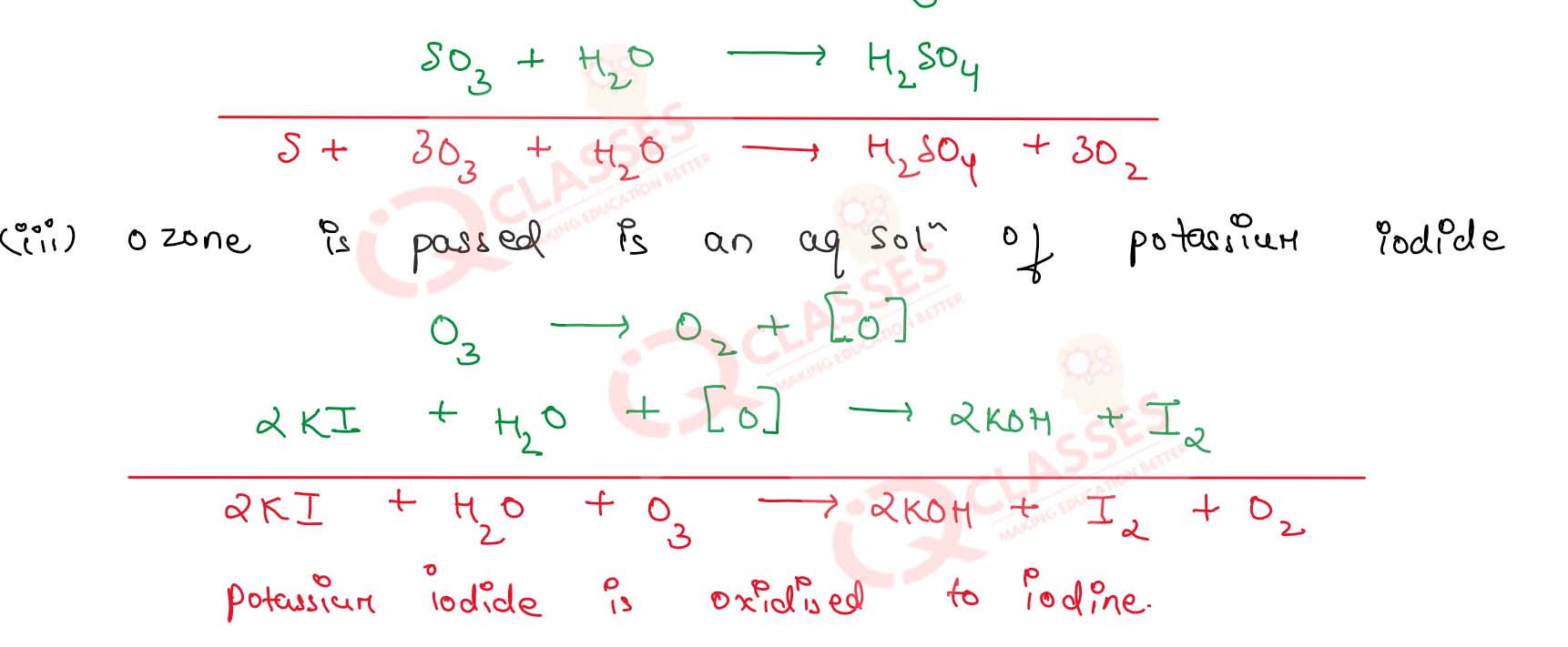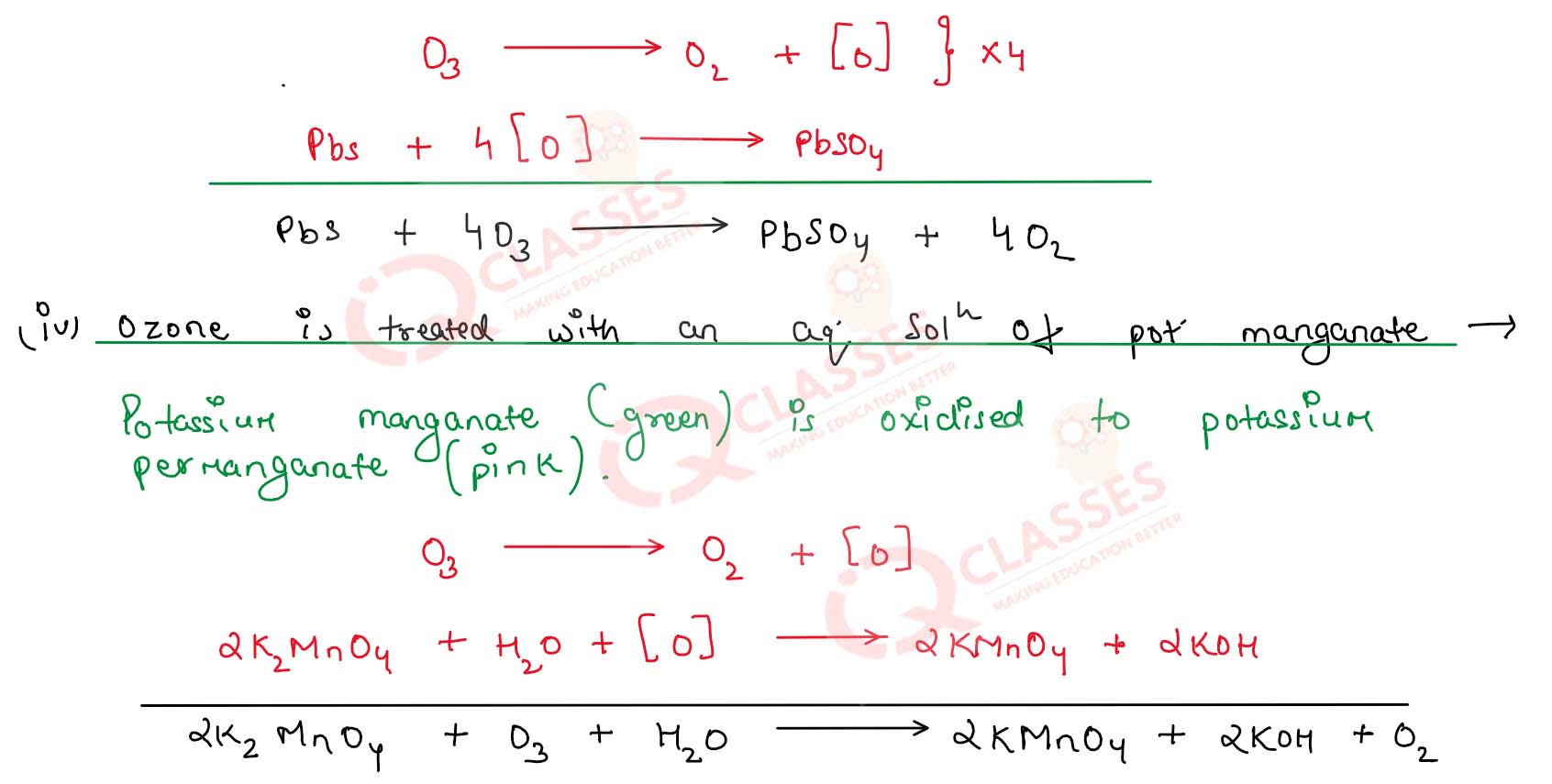7.1
Why is dinitrogen unreactive at ordinary temperature ?
Solution
Dinitrogen is reactive at room temperature is due to high
Stability of N≡N Molecules.
The N≡N has very high bond enthalpy (941.4 KJ).
7.2
What happens when :
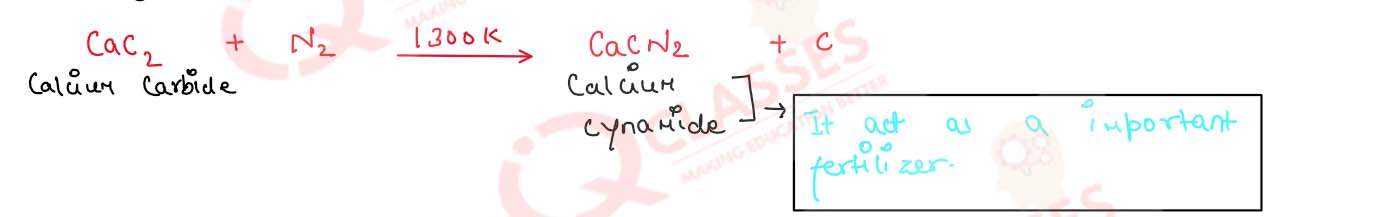
(i) Dinitrogen is passed over calcium carbide at 1300 K
(ii) Magnesium nitride is treated with water
(iii) Ammonia is treated with excess of chlorine
(iv) Nitric acid is poured over sawdust
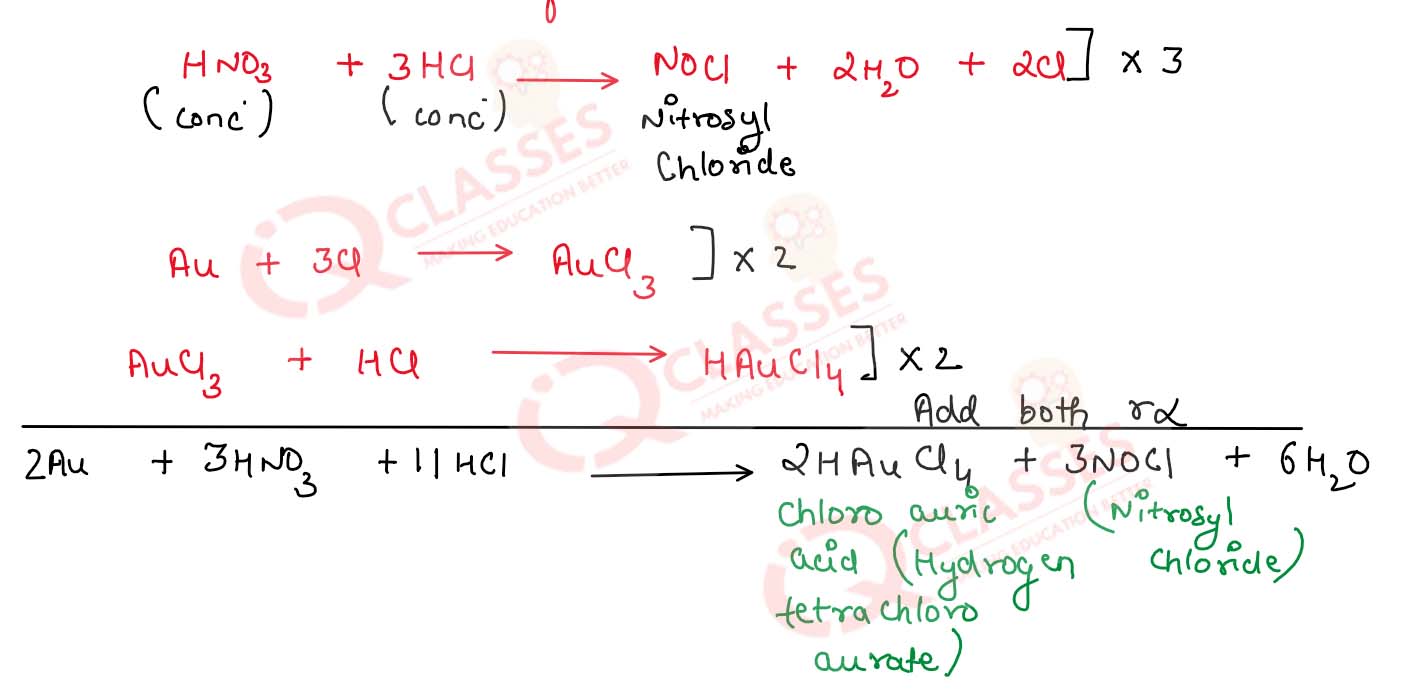
(v) Nitric acid is treated with gold.
Solution
(i) Dinitrogen is passed over Calcium Carbide at 1300K
(ii) Magnesium Nitride is reacted with water
Magnesium reacts with dinitrogen form Magnesium Nitride

Then Magnesium Nitride reacts with water form Ammonia

(iii) Ammonia is treated with excess of chlorine
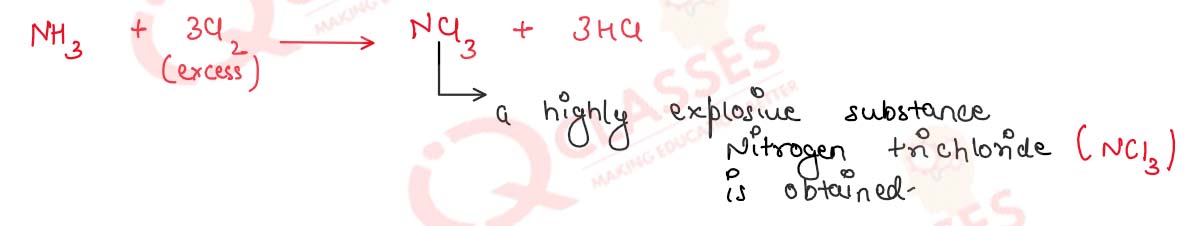
(iv) Nitric acid is poured over saw dust
when nitric acid is dropped over heated saw dust it catch fire spontaneously i.e. bursts into flame
(v) Nitric acid is treated with gold
chloroauric acid is formed
7.3
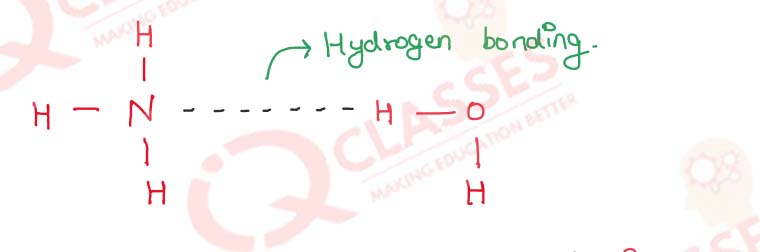
Why is solubility of ammonia in water is very high ?
Solution
Ammonia is highly soluble in water due to hydrogen bonding
7.4
Why is iron rendered passive in concentrated nitric acid ?
Solution
Formation of reddish brown Layer of iron oxide formed
Iron reacts with Conc. HNO3 , NO2

Highly conc. nitric acid (80%) makes iron passive.
7.5
Explain why
(i) A precipitate of silver chloride dissolves in NH4 OH;
(ii) Zinc sulpate does not give a precipitate with excess of NH4 OH;
(iii) Nitric acid makes wool yellow.
Solution
(i) The precipitate of silver chloride dissolves in NH4OH>

(ii) zinc sulphates does not give a precipitate with excess of NH4OH
with excess of NH4OH the ppt dissolves to give a Colourless solution of tetramine zinc (II)
sulphates

(iii) Nitric acid makes wool yellow
The yellow Strains on the wool fibres are caused due to the reaction of nitric acid with protein Keratin
present in the wool fibres
This reaction as called Xanthoproteic reaction.
7.6
Why does oxygen not show oxidation states of +4 and +6, Whereas sulphur does so?
Solution
Does not have vacant 2d orbital in their valence shells
In their ground state they possess only two-unpaired
e- which can fore only two bonds,
But in Sulphur shows
+ 4 and +6 oxidation State due to the presence of
vacant 3d orbital to which electron Can be promoted from 3s & 3p filled orbitals.
7.7
What type of hybridization is involved for oxygen in H2O. give reason for your answer
Solution
In Hybridisation of H2O the Oxygen atom is sp3 hybridized.
formation of water molecule there are three 2p orbitals and one 2s orbital 9 combine & formed four
sp3 orbitals.

7.8
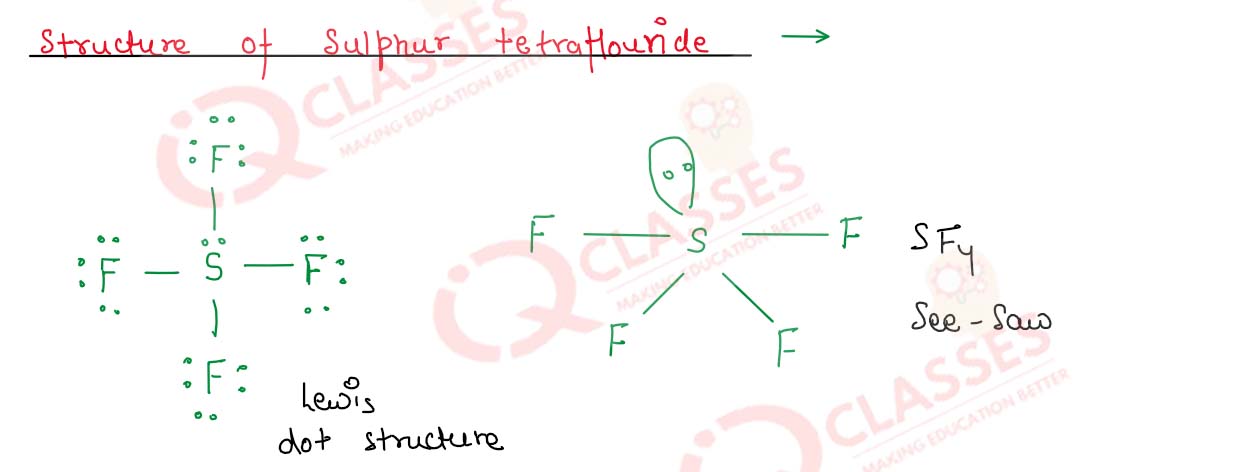
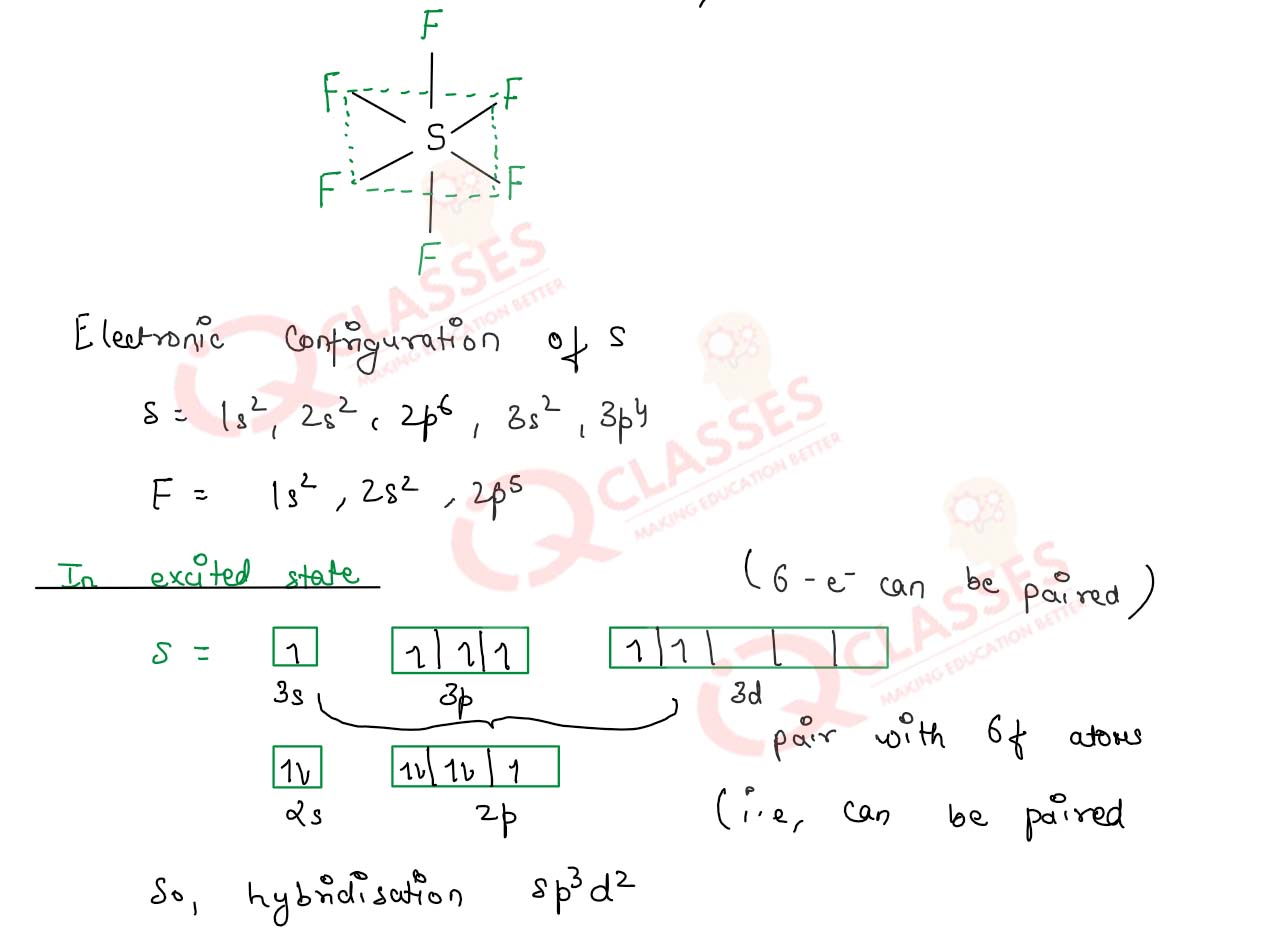
Give the structure of sulphur tetrafluoride
Solution
7.9
Discuss briefly the properties of group 16 elements define catenation and illustrate with example.
Solution
Properties of Group 16 elements->
[A]
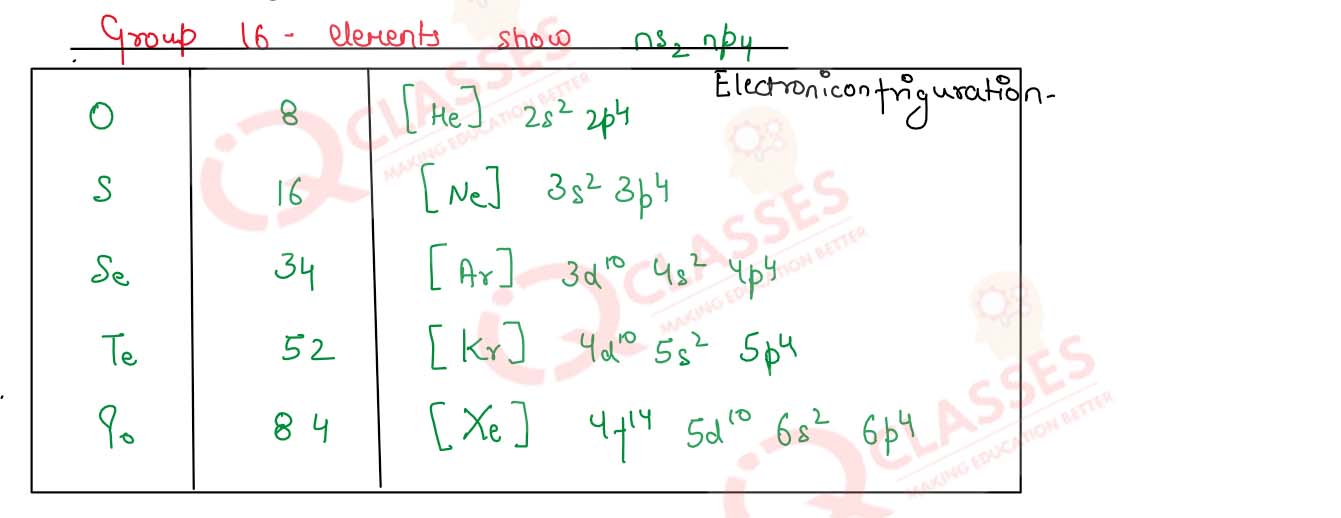
Electronic Configuration
[B] Atomic and Physical properties
(i) Physical state and Molecular structure
oxygen is the 1st member of Group-16 is a gas where as other element of the group Solid
(ii) Atomic Radius
As we go down the group
number of shells increase radii increase size also increase
Po> Te> Se> S>O
Increasing order of Atomic Radius
Ionization Enthalpy or Ionization Energy
Size ∝1/I.E
we go down the group the I. E decrease
then the Oxygen has more I.E among all the elements of group 16.
Electronegativity
As we move from left to Right Electronegativity increase
But, we go down the group , Electronegativity decrease.
Catenation
Oxygen the 1st member of the Group, show only a Little tendency towards Catenation
Example-> H-O-OH,[-O-O-]2- etc
sulphur shows greater tendency towards Catenation
Example->sulphanes,polysulphuric acid,polysulphides
7.10
Express H2O , H2S , H2Se , H2Te in decreasing order of
Thermal stability
Solution
H2O > H2S > H2Se > H2Te
decreasing order of Thermal
stability
7.11
Explain the hybridization in SF6 molecule what is the shape of this molecule ?
Solution
In SF6 Molecule the hybridisation is sp3d2
Sulphur Hexa flouride is
a type of greenhouse gas which is colourless , odourless and non-toxic .
7.12
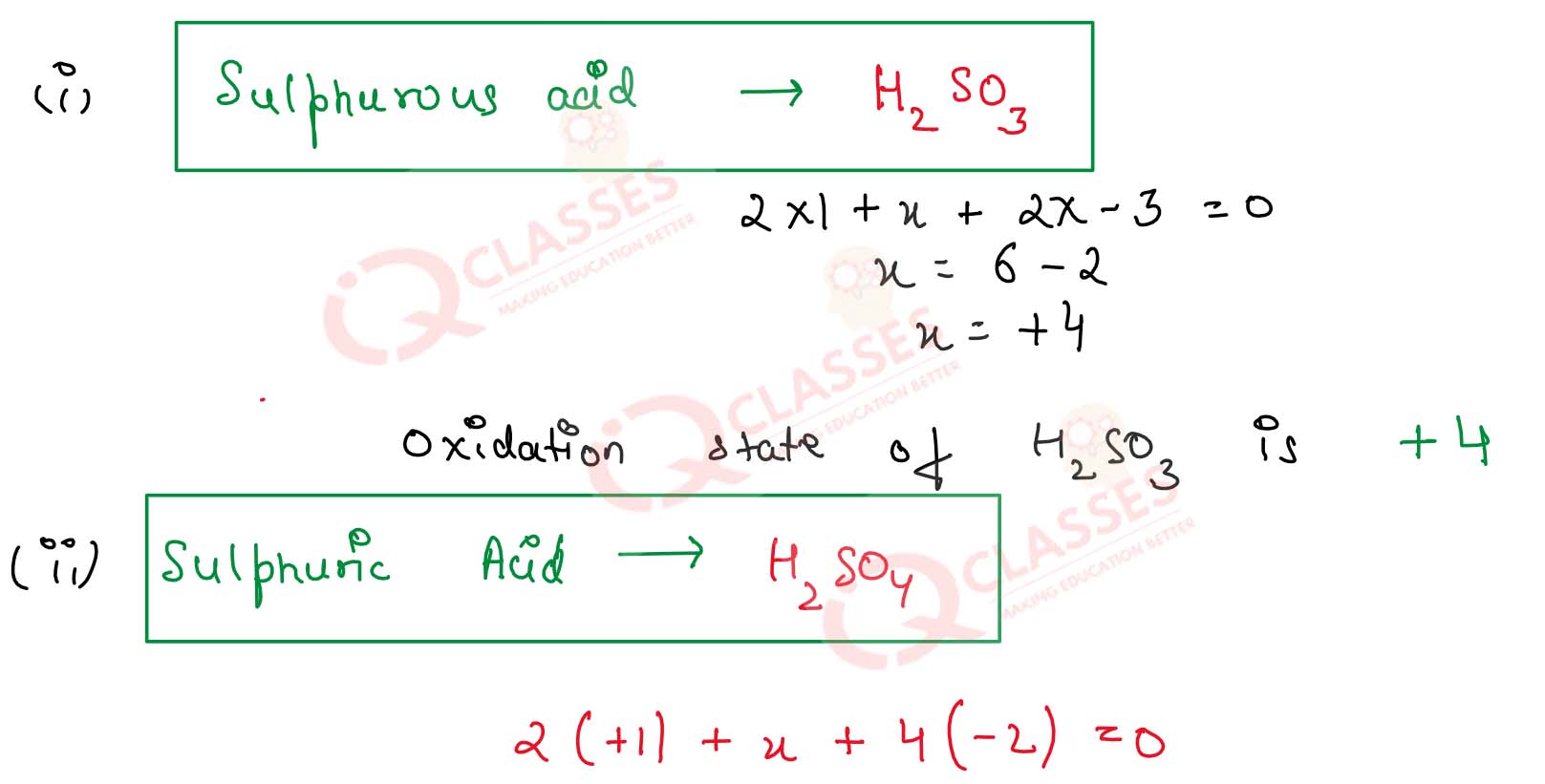
What is the oxidation state of S in the following oxoacids ?
(i) Sulfurous acid
(ii) sulphuric acid
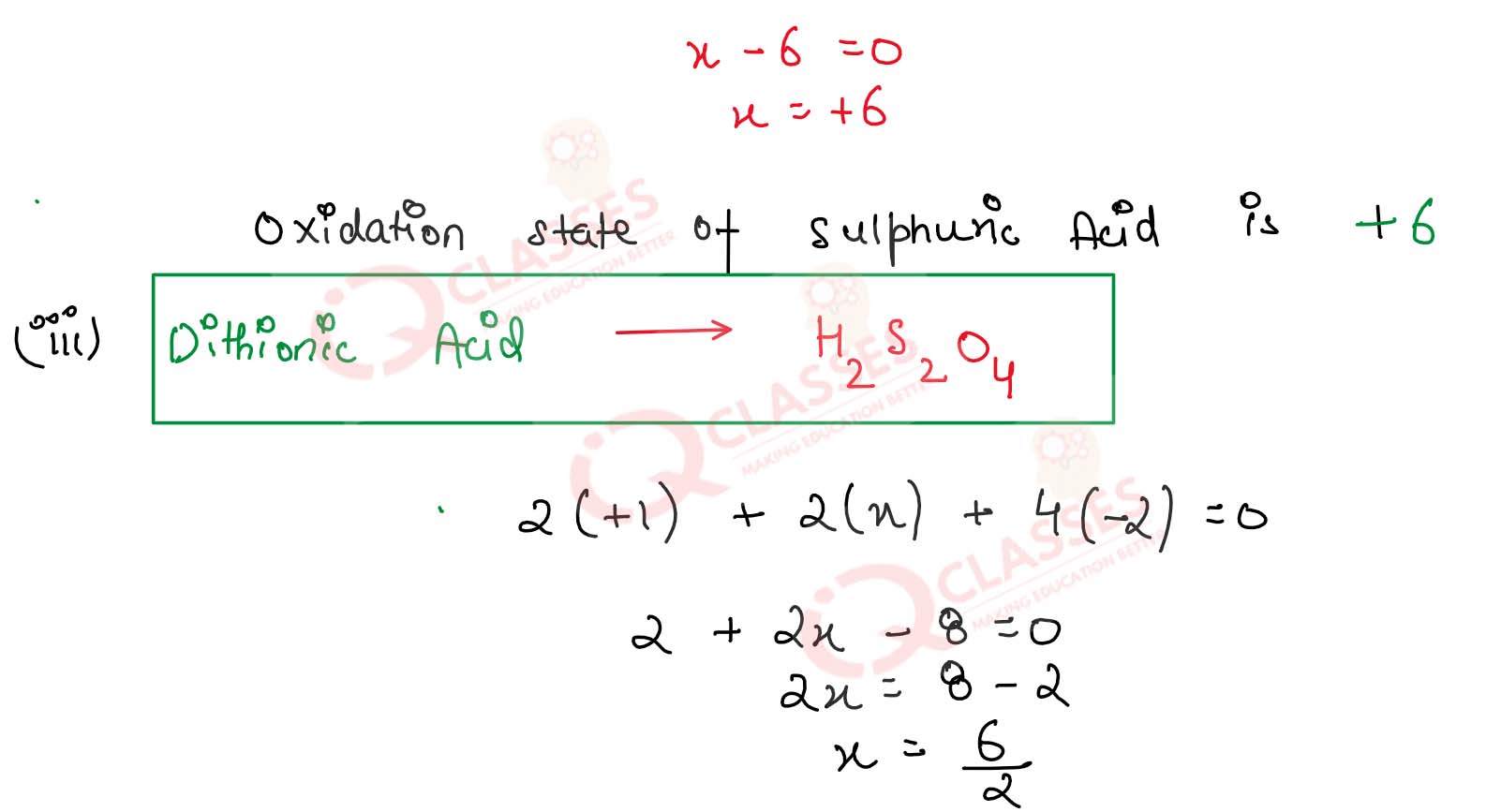
(iii) Dithionic acid
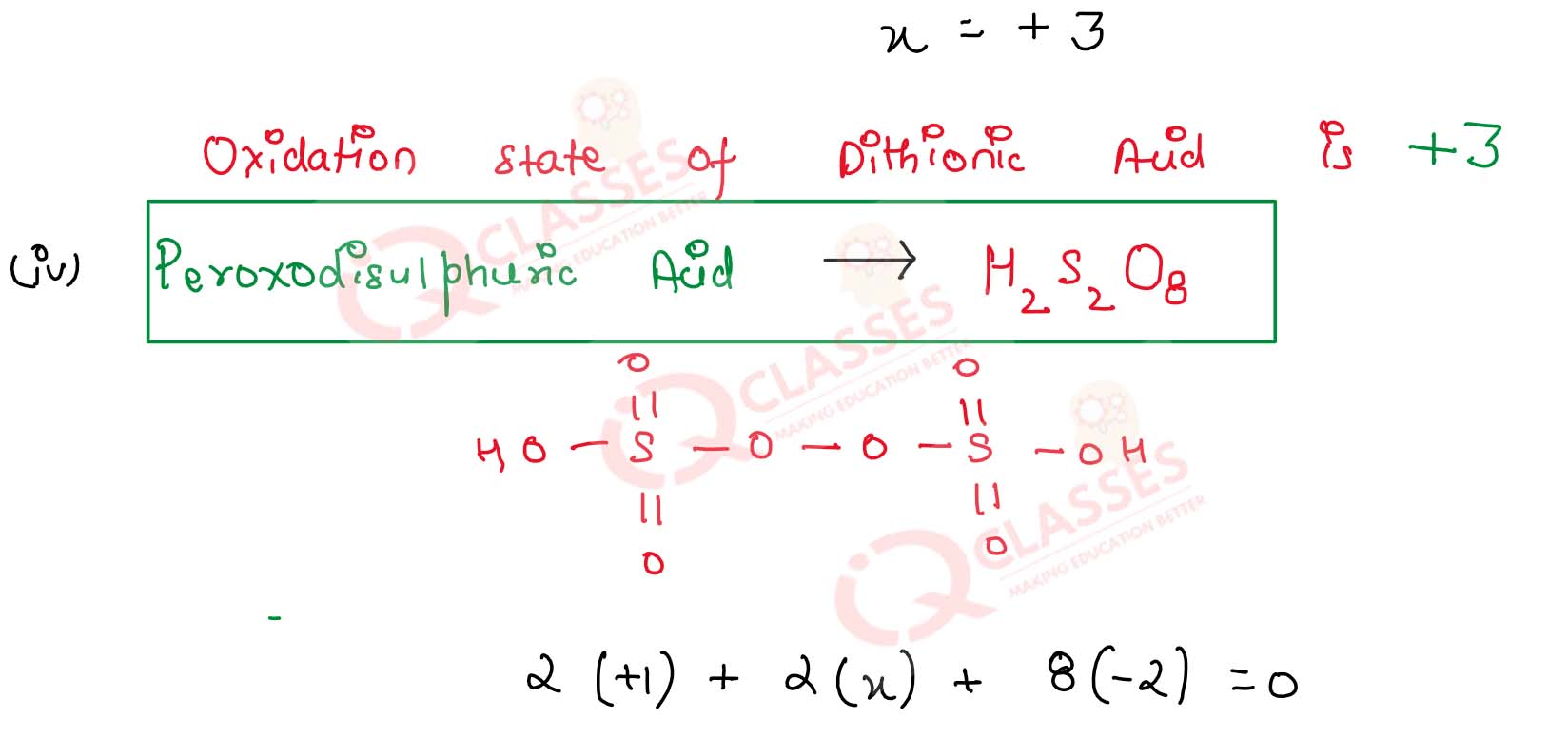
(iv) Peroxodisulphuric acid
Solution
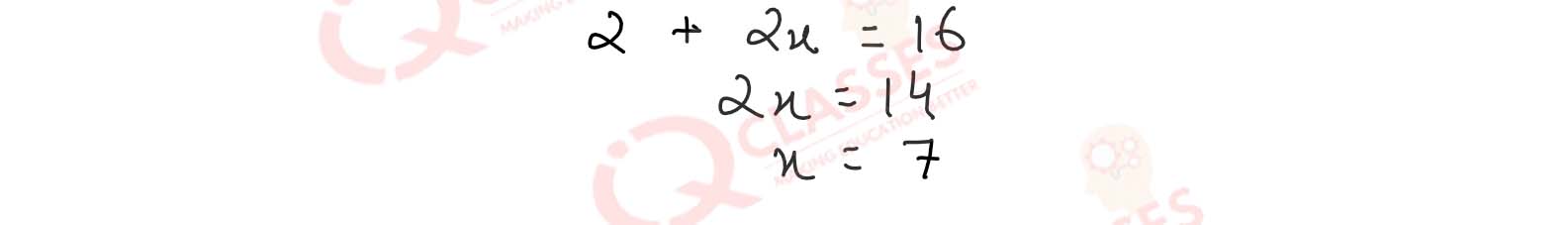
7.13
What type of hybridisation does explain the diagonal bipyramidal shape of SF4 >?
Solution
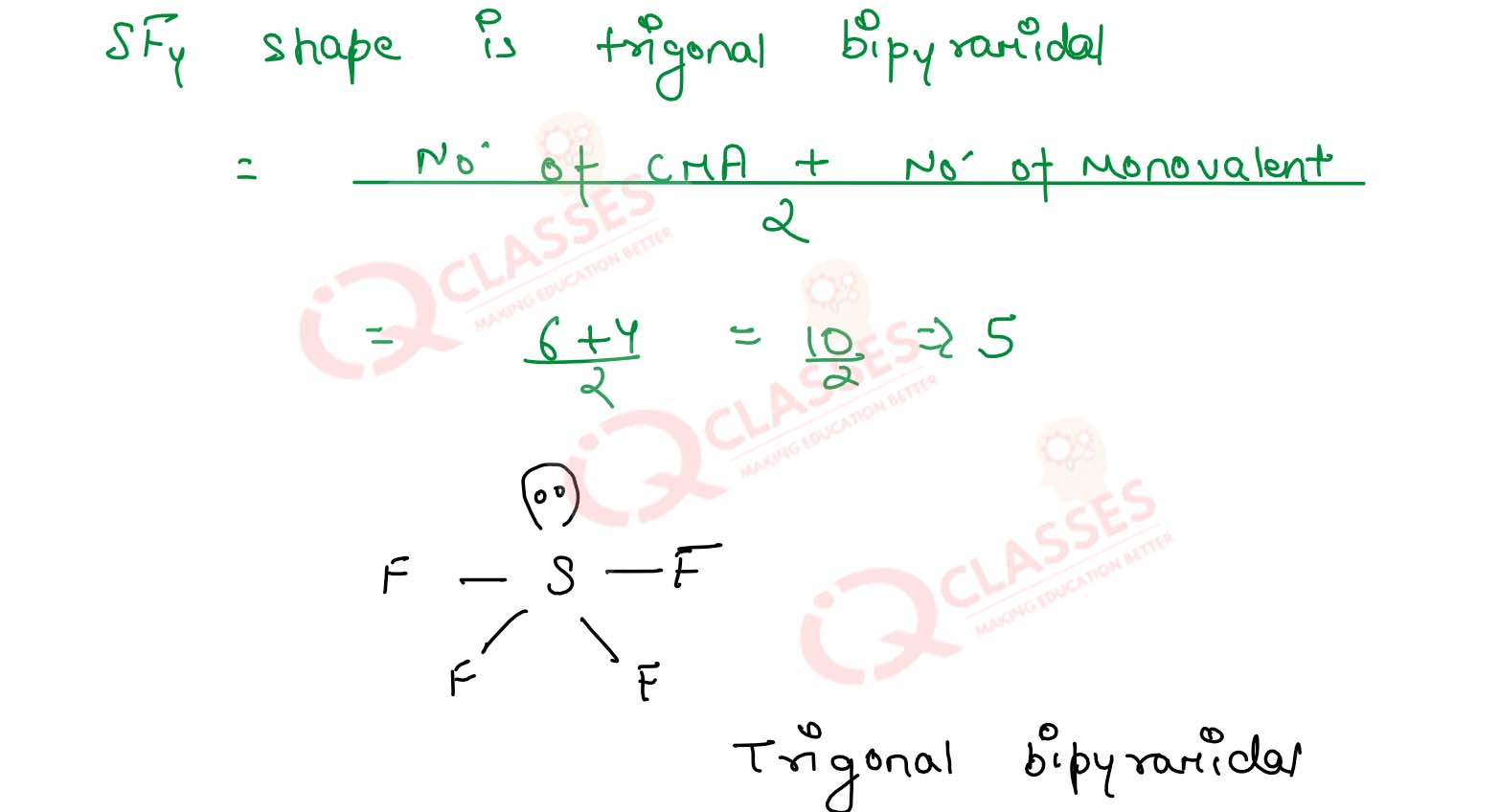
7.14
Describe the properties of O , Se , S , Te , Po (group 16 elements) with reference to
(i) Metallic / non metallic character
(ii) Catenation
(iii) Thermal stability of hydrides
(iv) Oxidation states
(v) allotropy
Solution
(i) Metallic and Non. Metallic character
Metallic character of
group 16 elements increase down the group
where as oxygen and sulphur are typical non-metals and insulator, Se Te are semiconductor and po is
metallic in nature.
(ii) Catenation
In group 16 Oxygen show that little tendency towards catenation
Example : link peroxides H-0-0-H [-0-0-]2- etc
but sulphur shows greater tendency towards catenation
(iii) Thermal stability of hydrides
The Thermal stability of Hydrides of group 16 elements
H2O > H2S > H2Se > H2Te
Thermal stability decrease
move from H2O to H2Te the size of the central atom increase.
(iv) Oxidation State
1. The most common oxidation state is -2.
2. sulphur can exists at +4 and +6 state.
3. For Se, Te, Po oxidation state +2 , +4 +6 are
possible
4. Oxygen does not show +4 and +6 oxidation state due to presence of d-orbital.
(v) Allotropy
Group 16 -elements
For Oxygen : it is exists as dioxygen (O2) and Ozone (O3)
For sulphur: Homocyclic species containing 6-20 sulphur atom,
It is also exits as chain polymer (Catena-Sn sulphur).
For Selenium : it is exists six allotropic modification
three are α , β, and γ form are red
monoclinic form and Contain S8 rings.
The most stable form grey Hexagonal monoclinic form
For Te and Po : it also show allotropy and Po has two
allotropic α-form and β-form
7.15
Draw the molecular structure of peroxomonosulphuric acid
Solution
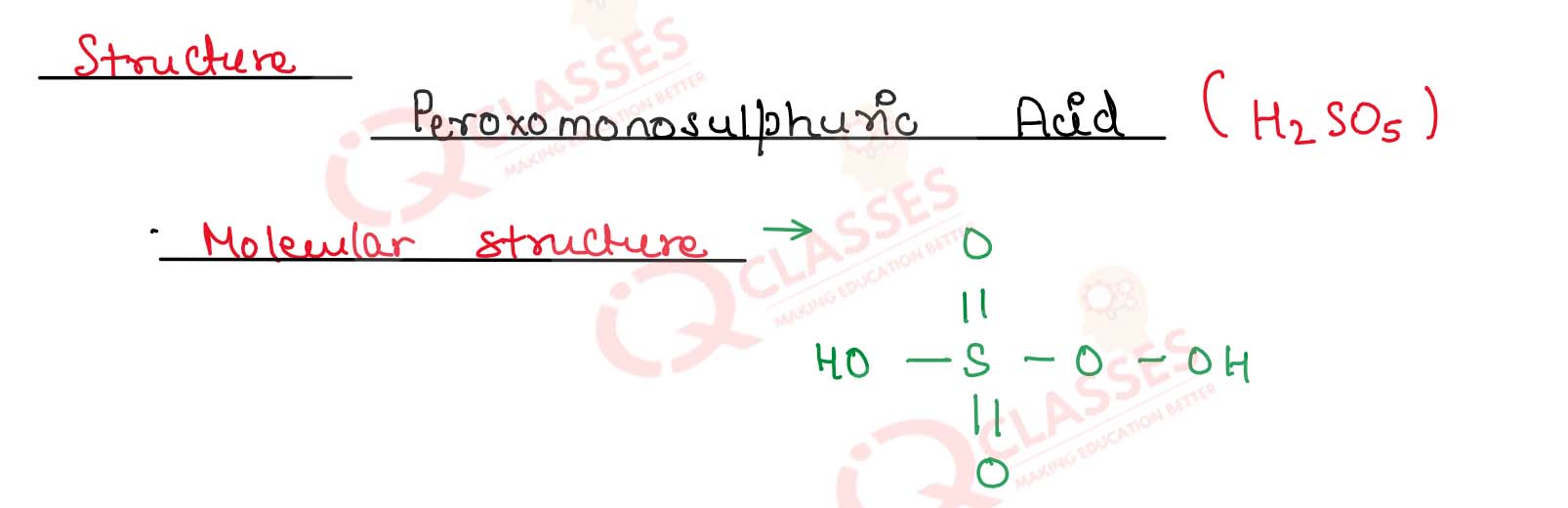
7.16
Which has the larger bond angle H2S or H2S and why?
Solution
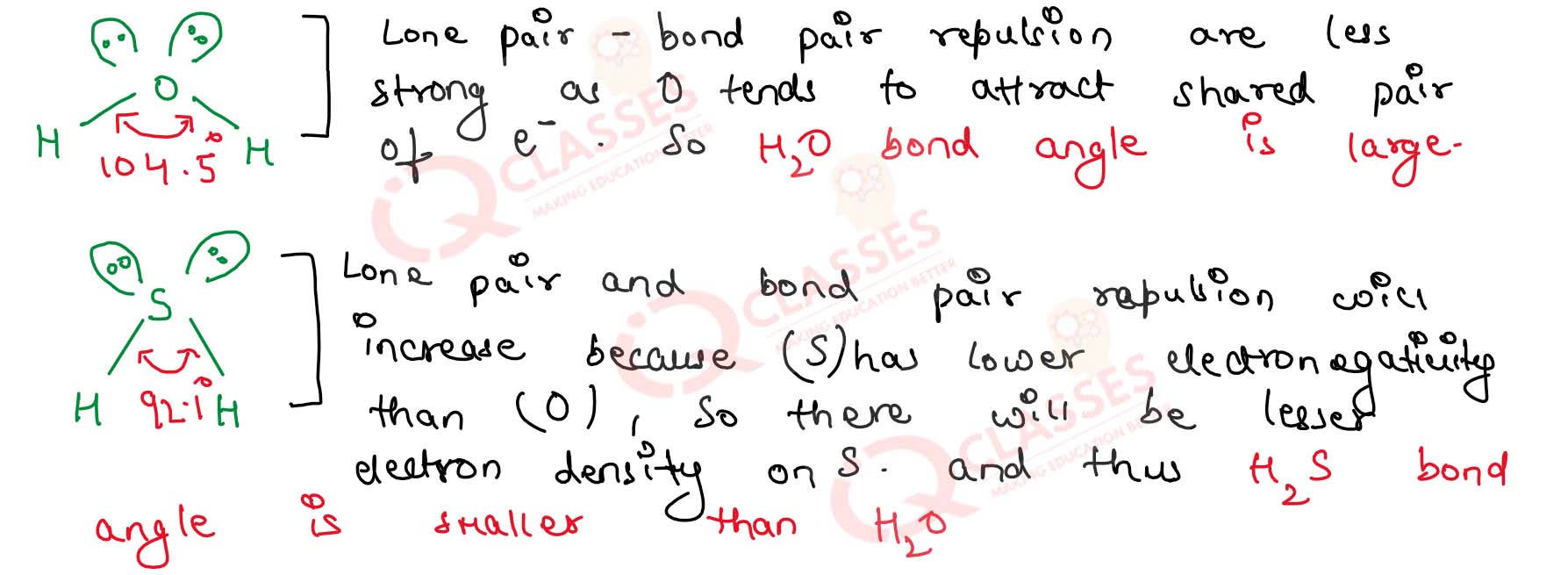
5.17
Explain the following observation :
Among the hydrides of elements of group 16 water shows unusual
physical properties
Solution
water shows unusual physical properties such as
high Boiling point , weak acidic character & High B.P. as compared to the other Hydrides of group-16.
7.18
Why does sulphur in vapour state exhibit paramagnetic behaviour ?
Solution
In vapour state sulphur exists as S2 molecule , which has two-unpaired electron in
antibonding pie orbital, same as O2 molecule Hence its exists as paramagnetism.
7.19
Account for the following :
(i) Tendency to show -2 oxidation state diminishes from sulfur to polonium in Group 16
(ii) NO2 readily forms a dimer whereas ClO2 does not
Solution
(i) The valence shell electronic configuration of group 16 ns2np4
strongly electronegativity these elements prefer to
gain two electrons to attain noble gas configuration and thus exhibit an oxidation State of -2.
(ii) NO2 forms dimer due to presence of odd electron whereas ClO2 does not form
dimer because due to the reason that odd electron is delocalised as it is involved in p(pi) -d(pi)
bonding.
7.20
Assign appropriate reason for each of the following observations :
(i) SF6 is not easily hydrolysed whereas SF4 is easily hydrolysed.
(ii) Sulphur in vapour state exhibits some paramagnetic behaviour
Solution
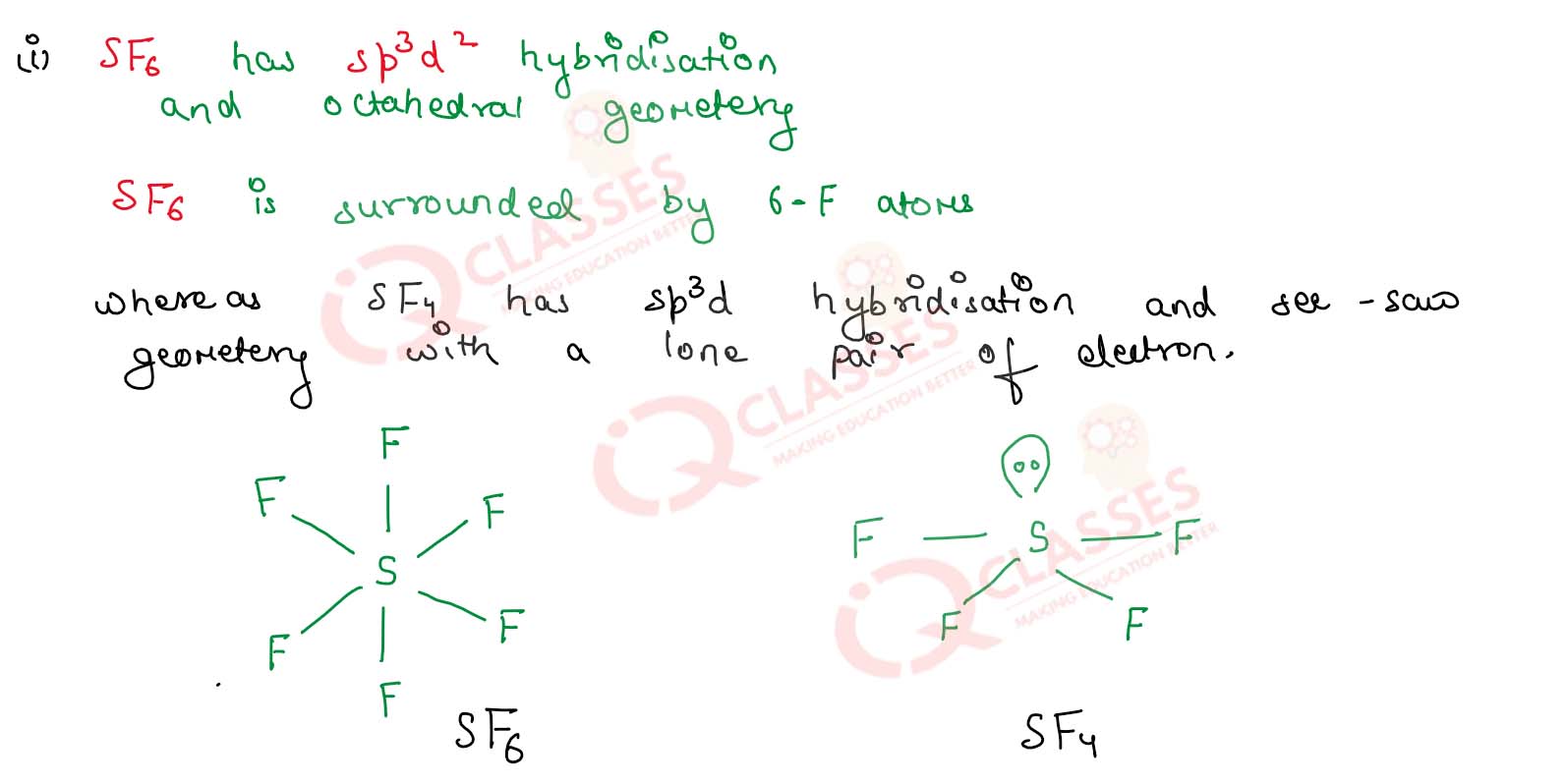
Due to the presence of six -flourine atoms in SF6 it is steric hindrance so water cannot
attack and SF6 is not easily hydrolysed.
Where as in SF4
the lone pair of electron and geometry so it is hydrolysed.
(ii) In vapour state sulphur exists as S2 molecule ,
which has two-unpaired electron in antibonding pie
orbital, same as O2 molecule Hence its exists as paramagnetism.
7.21
Give reason for the following statement :
SF6 is not easily hydrolysed though thermodynamically it should be
Solution

Due to the presence of six -flourine atoms in SF6 it is steric hindrance so water cannot
attack and SF6 is not easily hydrolysed.
Where as in SF4
the lone pair of electron and geometry so it is hydrolysed.
7.22
Give reason for the following facts :
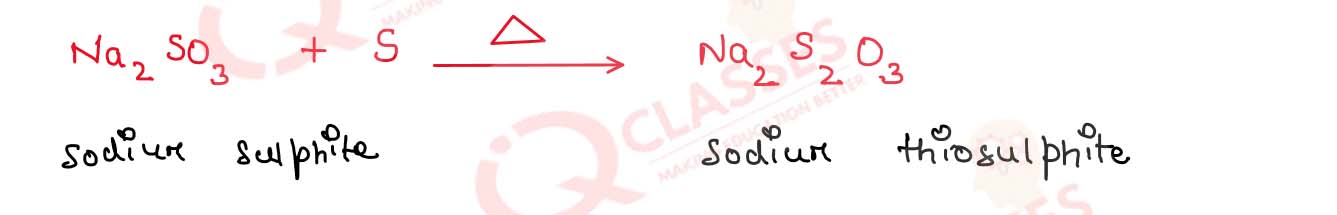
(a) Sulfur disappears when boiled with an aqueous solution of sodium sulphite
(b) H2 SO4 is diprotic acid
Solution
Sulphur disappear when boiled with an aqueous solution of sodium sulphite because Sodium thiosulphate is
formed
sodium thiosulphate is soluble in water and this disappearance easily in water
(ii) some strong acids such as sulphuric acid are diprotic acid that have two H+ which
dissociates one at a time.
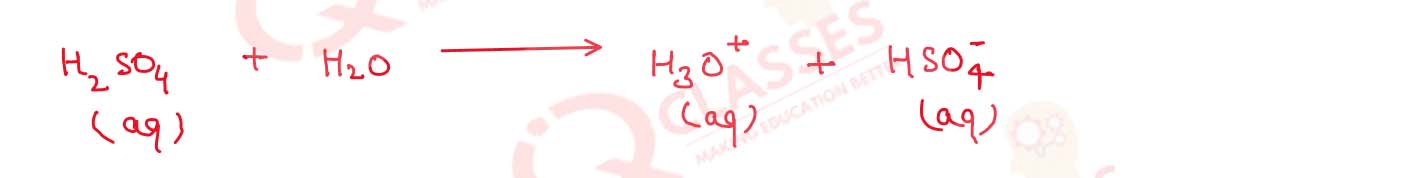
7.23
Assign a reason for the following :
Sulfur hexafluoride is used as a gaseous electrical insulator
Solution
Sulphur Hexafluoride used as electrical insulator is an good dielectric gas
for rich high voltage application
It is chemically inert,
non-flameable, non-corrosive , non - toxic.
7.24
Assign a reason for the following :
(i) SF6 is known but SCl6 is not known.
(ii) SF6 is not easily hydrolysed
Solution
(i)
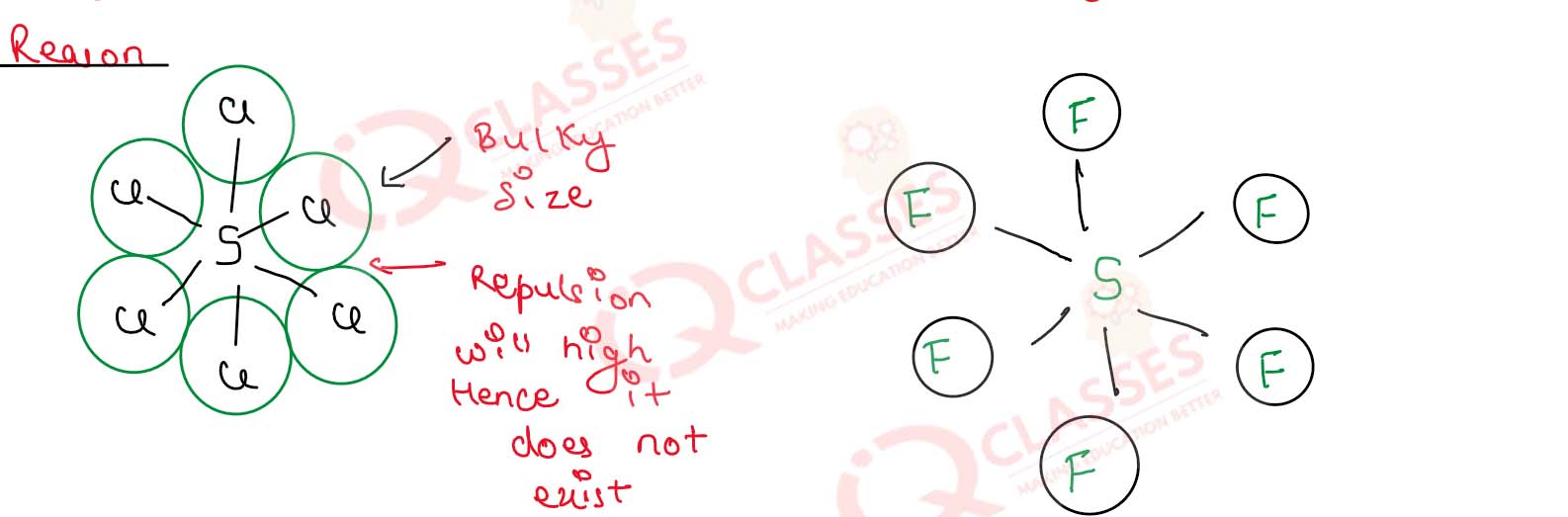
Flourine has smaller in size accommodate its six
atom in given space of sulphur while chlorine being bigger in size is not able to accommodate.
due to repulsion b/w its six atoms
SF6 is known but SCl6 is not known.
(ii)

Due to the presence of six -flourine atoms in SF6 it is steric hindrance so water cannot
attack and SF6 is not easily hydrolysed.
Where as in SF4
the lone pair of electron and geometry so it is hydrolysed.
7.25
Discuss the structures of SO2 and SO3
Solution
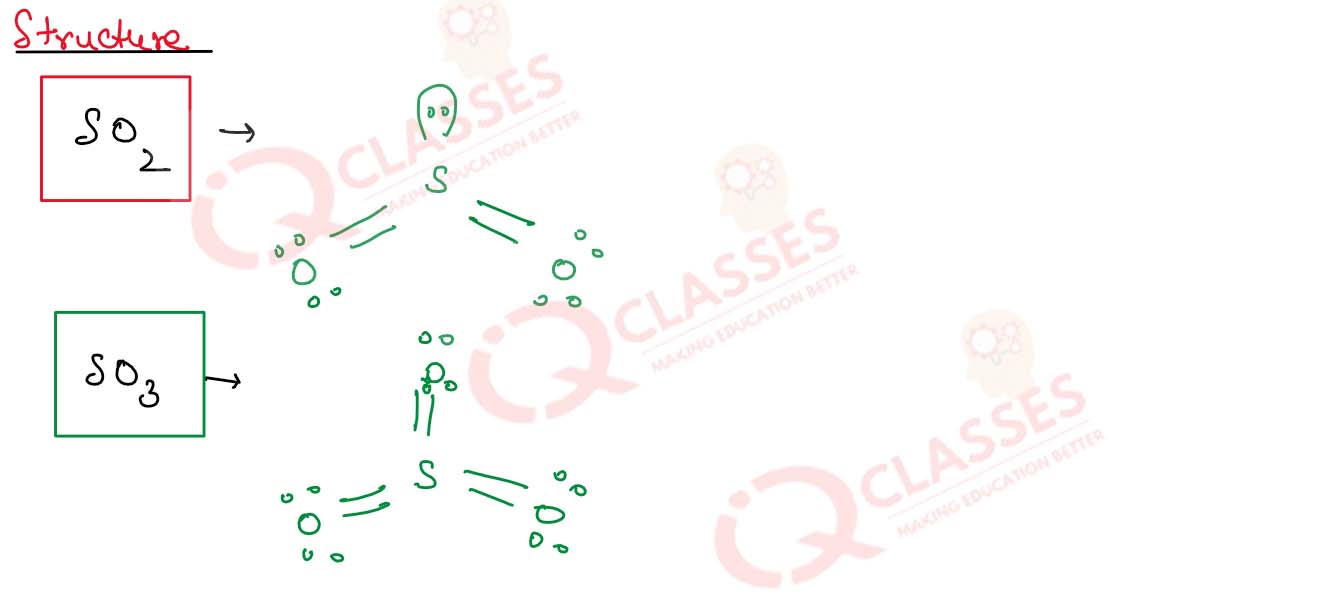
7.26
What is allotropy ? Why is ozone regarded as an allotrope of oxygen
Solution
Allotropy is the phenomenon of an element existing in two or more physical forms
different structural modifications of an elements.
-> ozone is an allotrope of Oxygen
-> It is 03 while normal oxygen is 02
-> ozone absorb harmful ultra-voilet (U.V.) rays from the Sun.
7.27
Why is ozone used as a disinfectant ?
Solution
It is used as an oxidising agent.
ozone is used as a disinfectant and germicide in the purification of water.

7.28
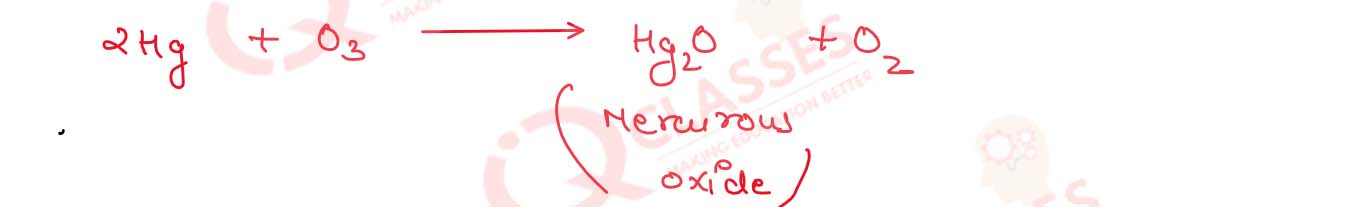
What is tailing of mercury ?
Solution
Tailing of mercury ->
(03) it is used as a Laboratory test to detect
the presence of ozone . ozone is an oxidising agent and its reacts with mercury to form H2O
due to which it losses its meniscus and sticks on the wall of the glass this is called tailing of
mercury
7.29
How is ozone found in the upper atmosphere and how is it useful for us ?
Solution
In the upper Layer of atmosphere the ultraviolent rays split the molecules of oxygen (02)
into its constituents two atoms.
Each of the atom then combine with another oxygen (02) molecule when gives
rise to Ozone (03)

7.30
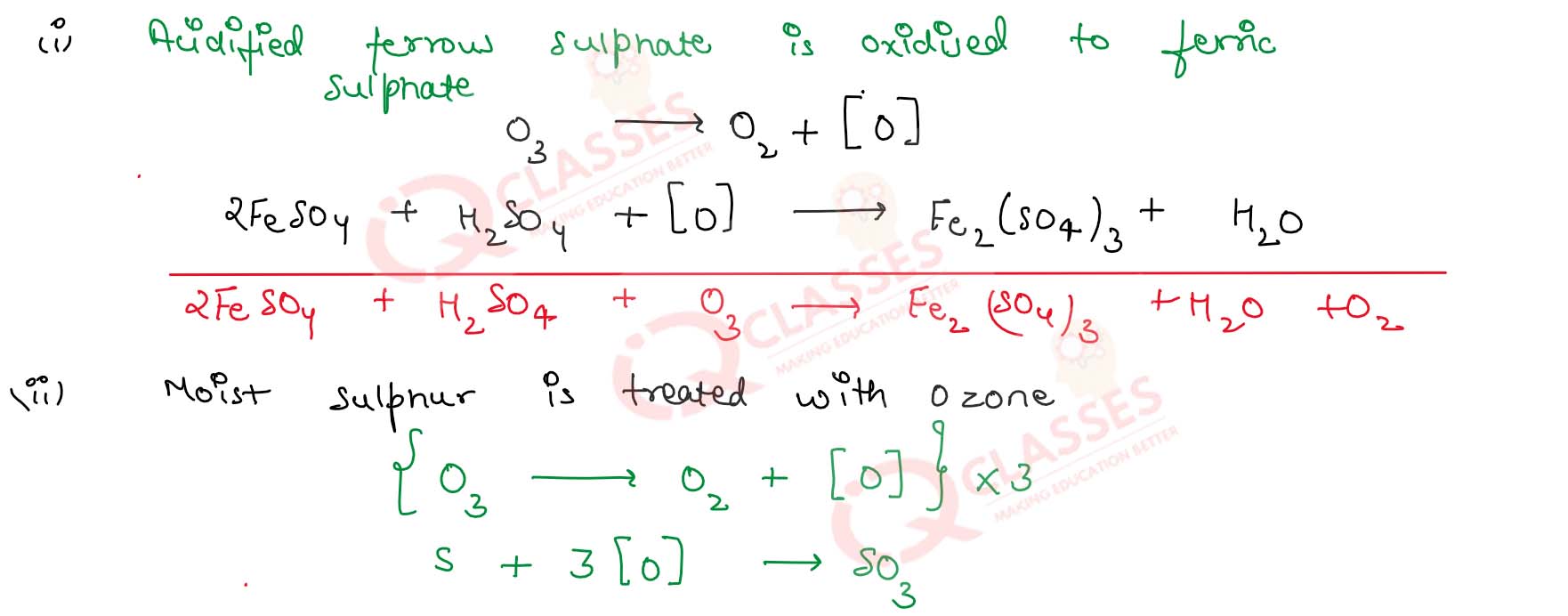
What happens when :
(i) Ozone is passed in an acidified solution of ferrous sulphate
(ii) Moist sulphur is treated with ozone
(iii) Ozone is passed in an aqueous solution of potassium iodide
Solution
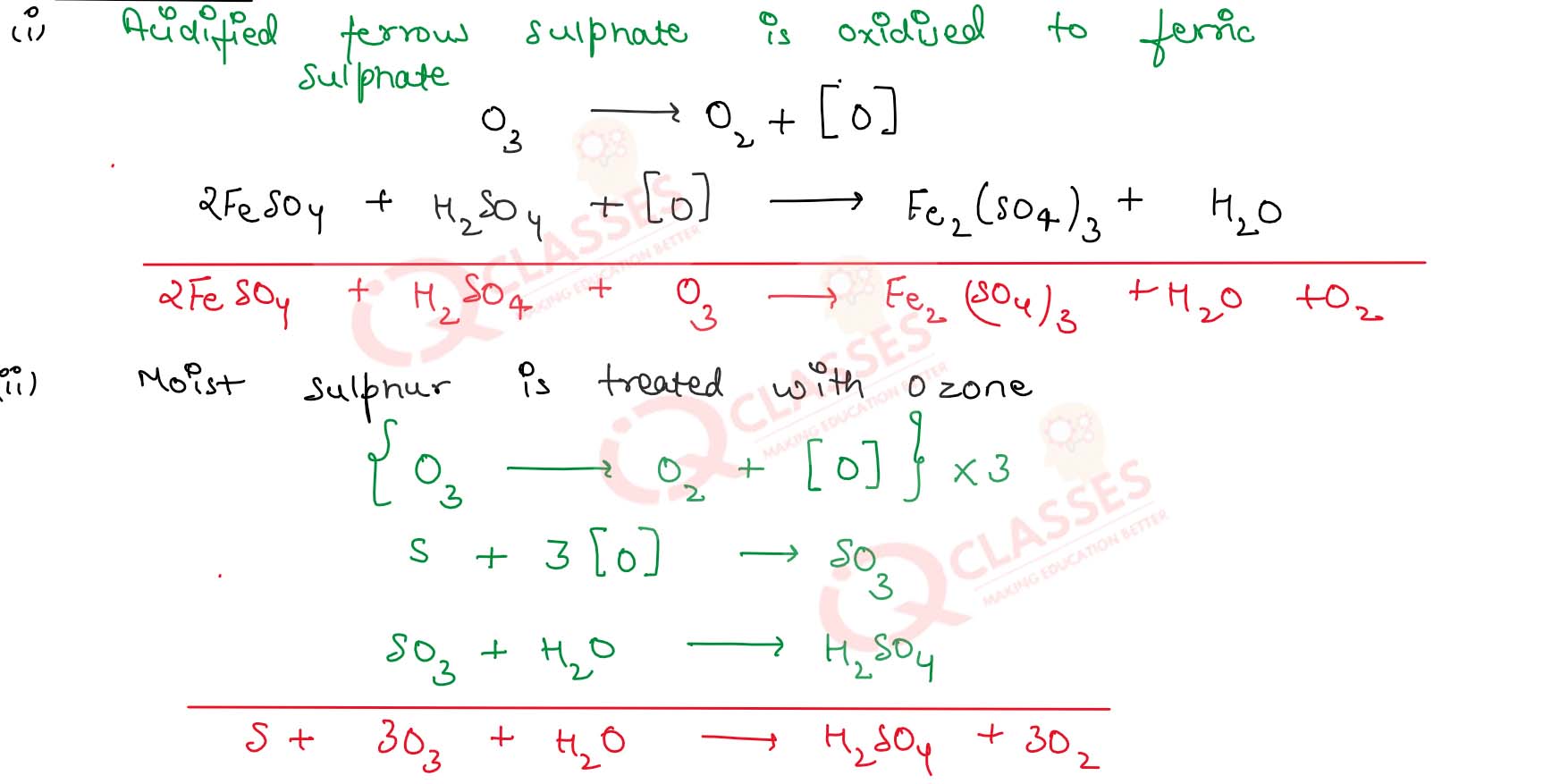
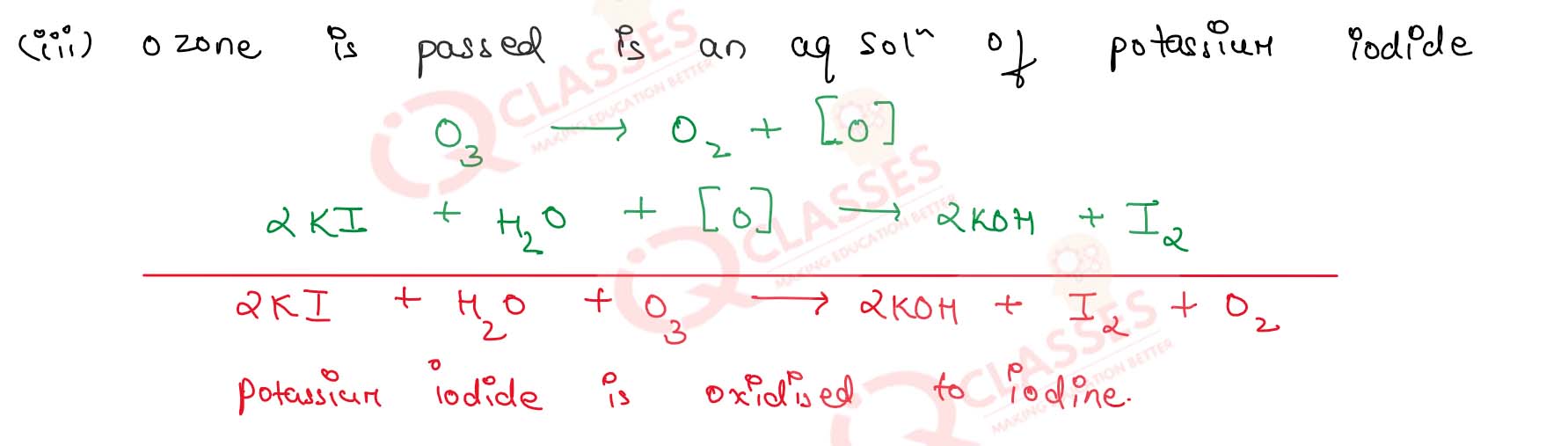
7.31
Out of oxygen and ozone which is better oxidising agent and why ?
Solution
ozone is a powerful oxidising agent because it
decompose readily under normal Condition form nascent oxygen which is very reactive radical and
responsible for the oxidation of number of substance
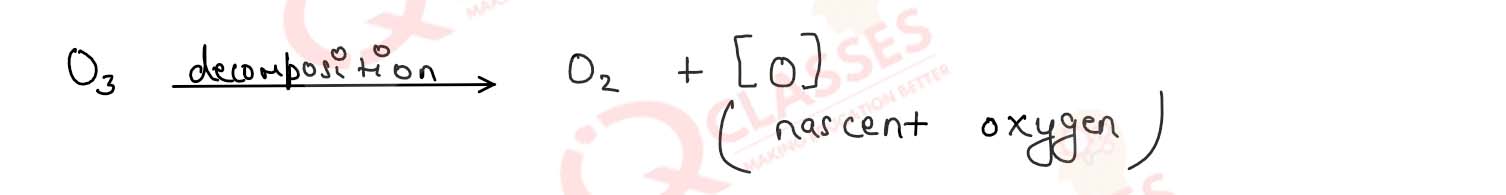
7.32
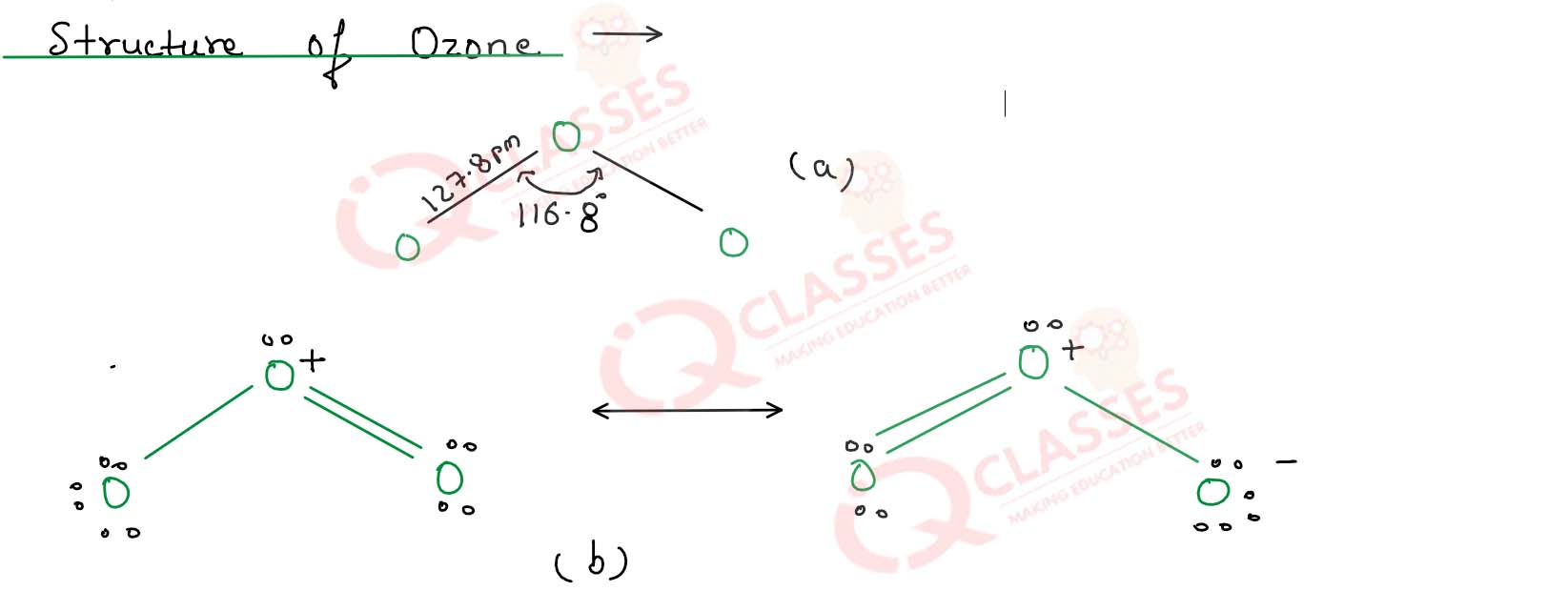
How is ozone prepared ? Discuss its structure
Solution
ozone is an allotropic form of oxygen and exists as a triatomic molecules.
Preparation of ozone->
Ozone is prepared by passing a silent electric discharge is pure and dry dioxygen.

This reaction is endothermic and formation of one mole of ozone involves in the absorption
The apparatus used for the preparation of ozone is called ozoniser.
7.33
Describe the important oxidation reaction of ozone
Solution
The important oxidation reaction of ozone are :
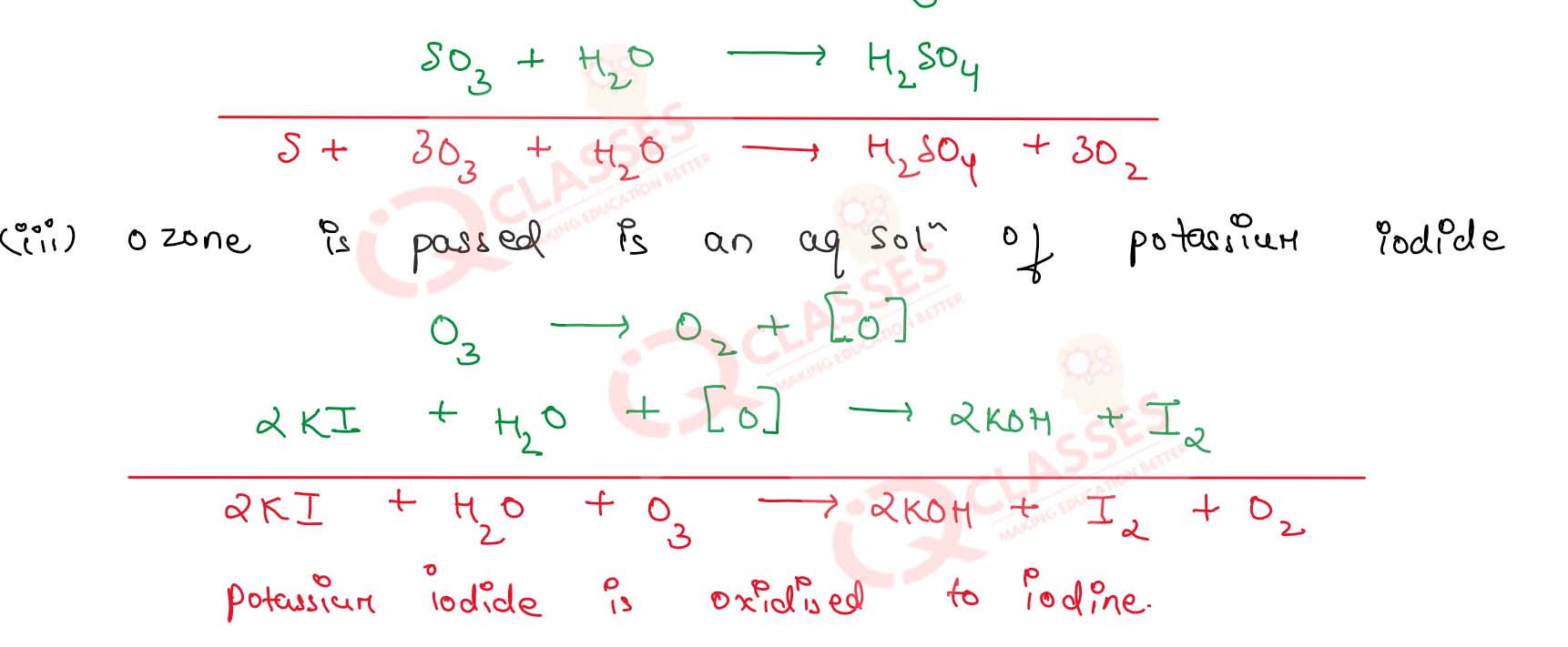
7.34
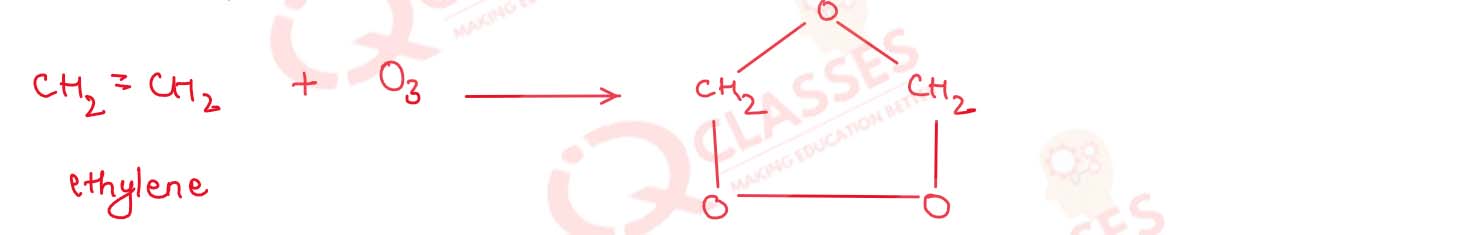
What happens when :
(i) Ozone is treated with ethylene
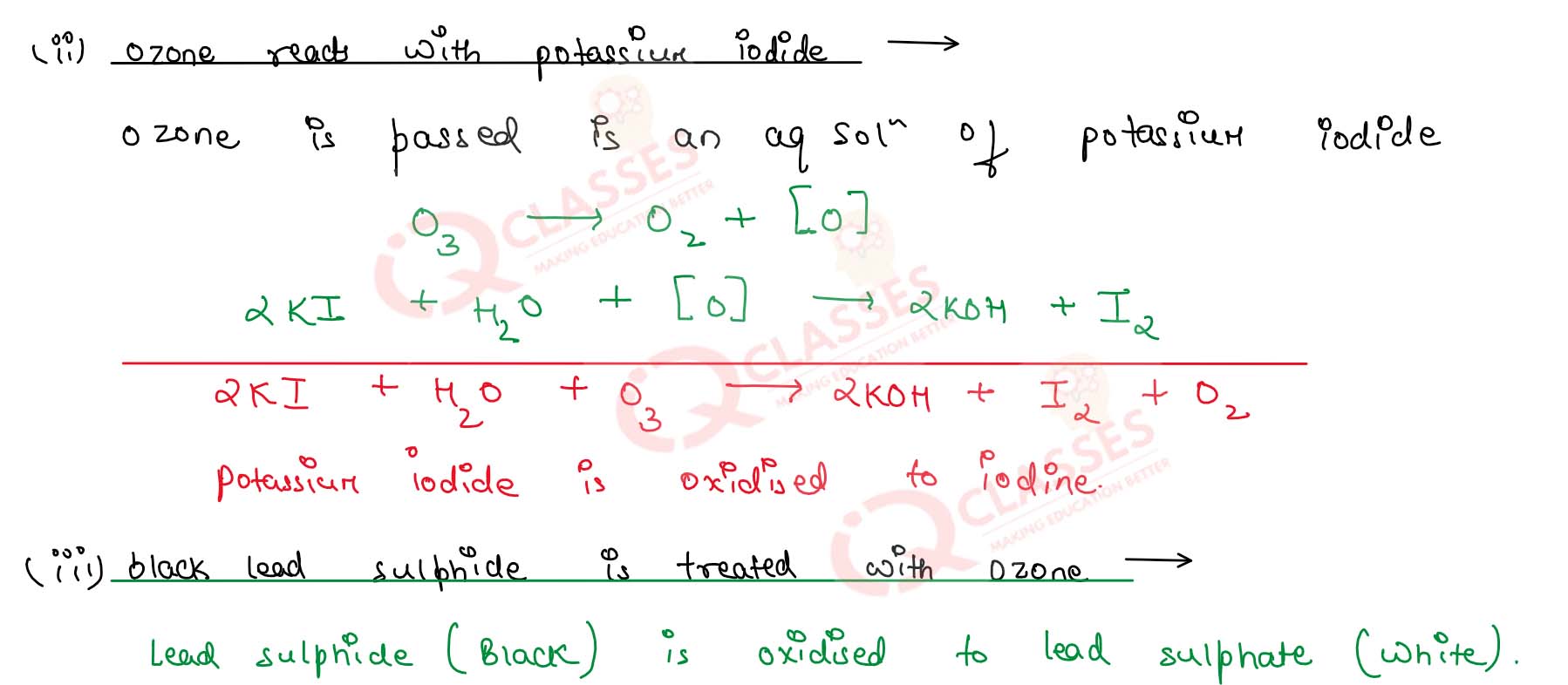
(ii) Ozone reacts with potassium iodide
(iii) Black lead sulphide is treated with ozone
(iv) Ozone is treated with an aqueous solution of potassium manganate ?
Solution
(i) Ozone is treated with ethylene :
When ozone reacts with unsaturated organic Compounds Containing double bonds to form addition products
called ozonoid.