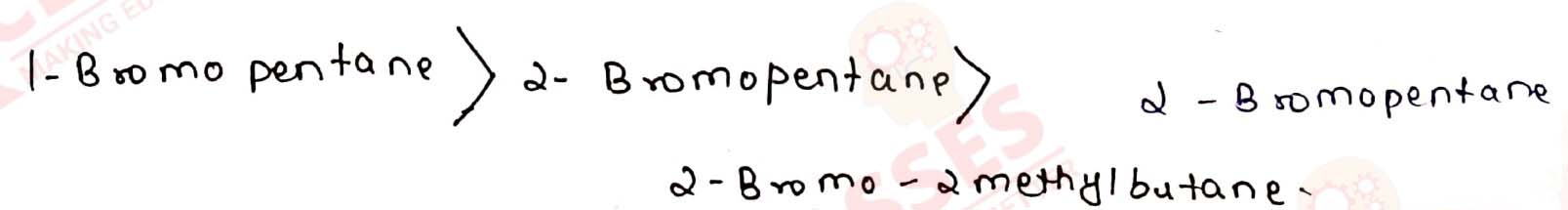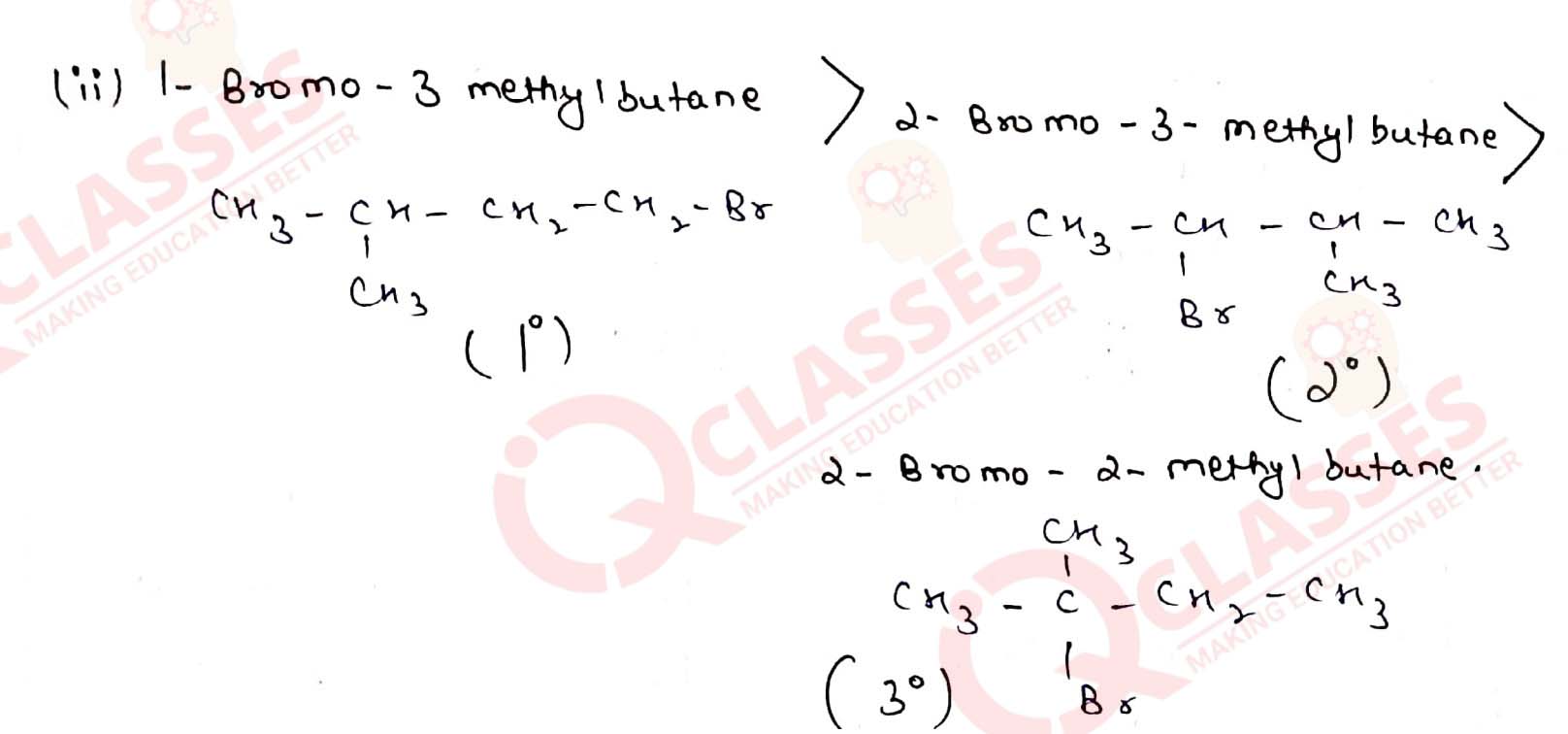Q10.1
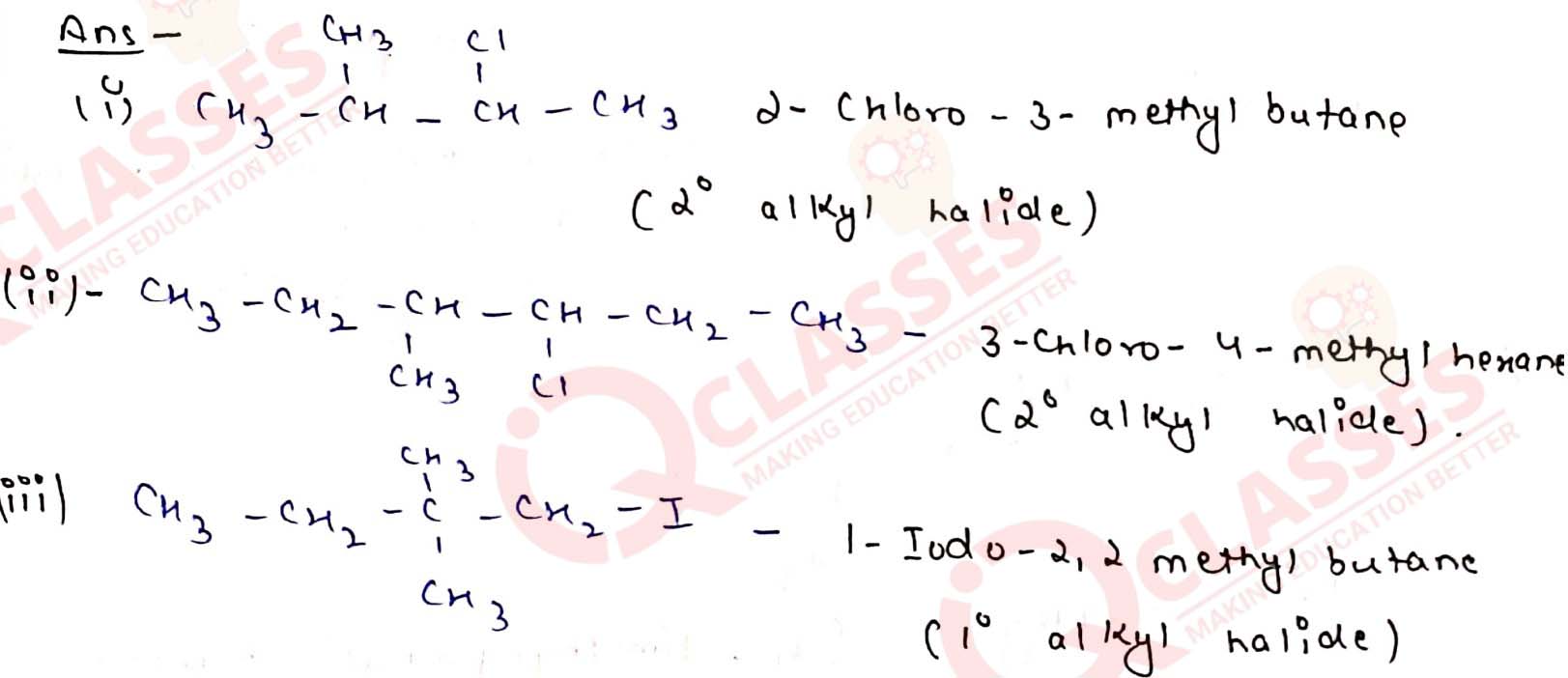
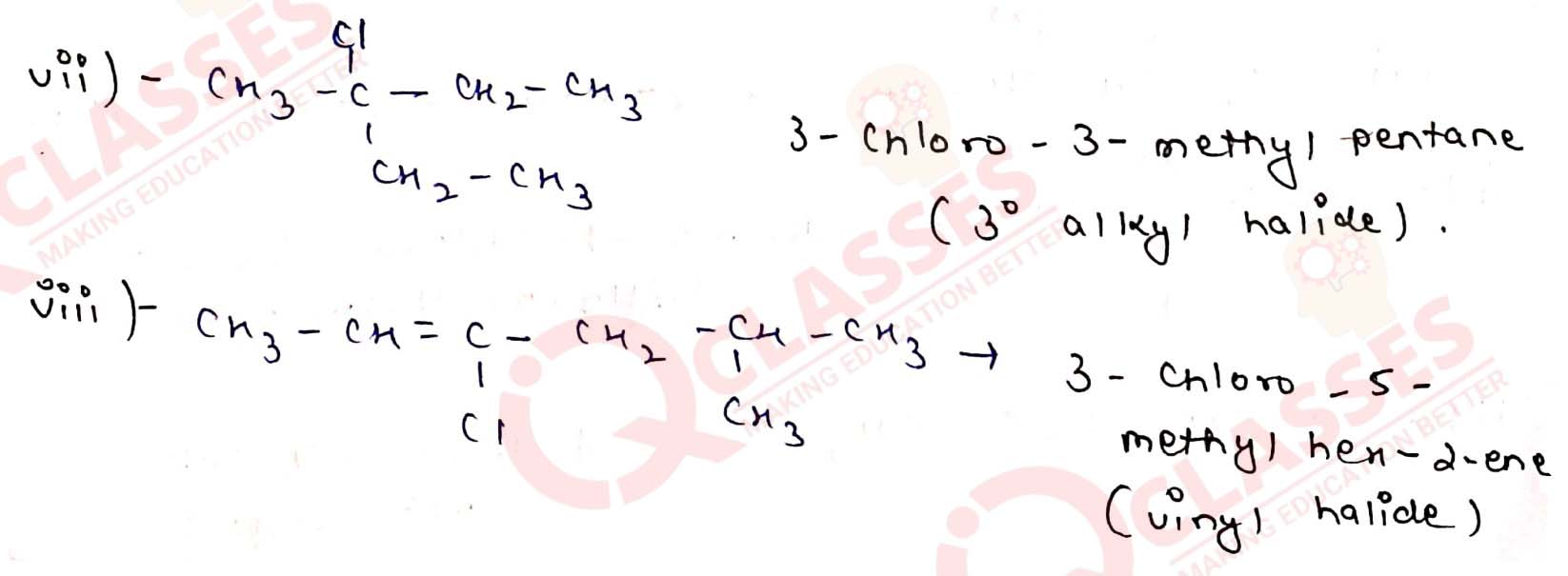
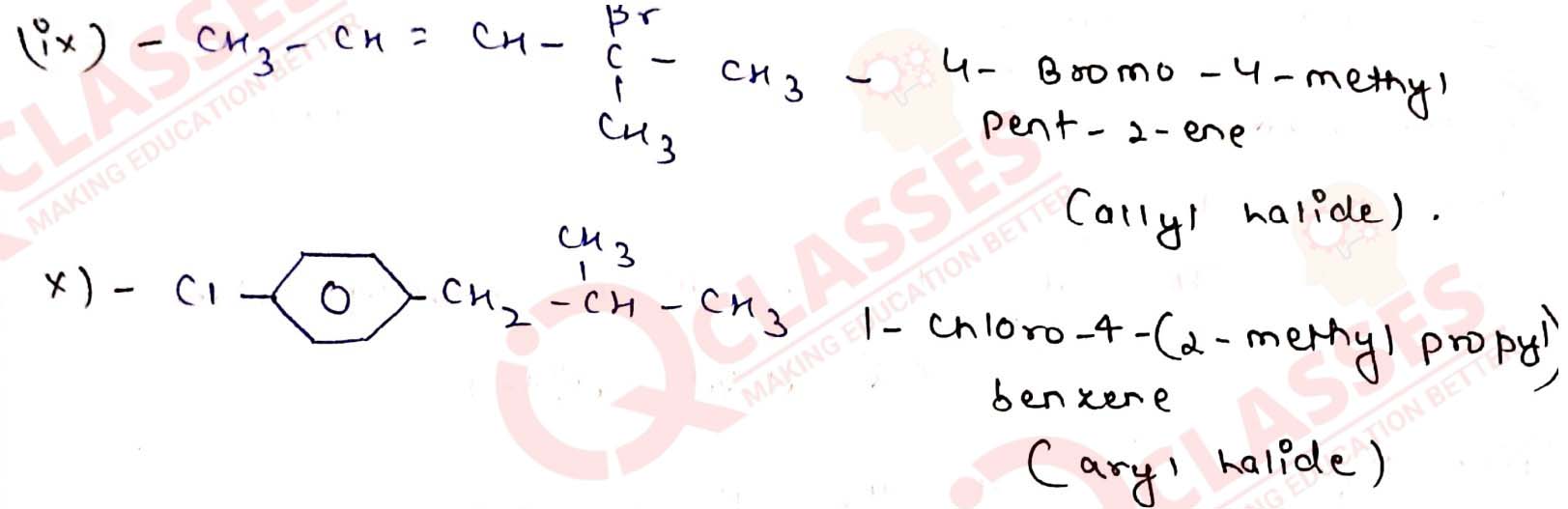
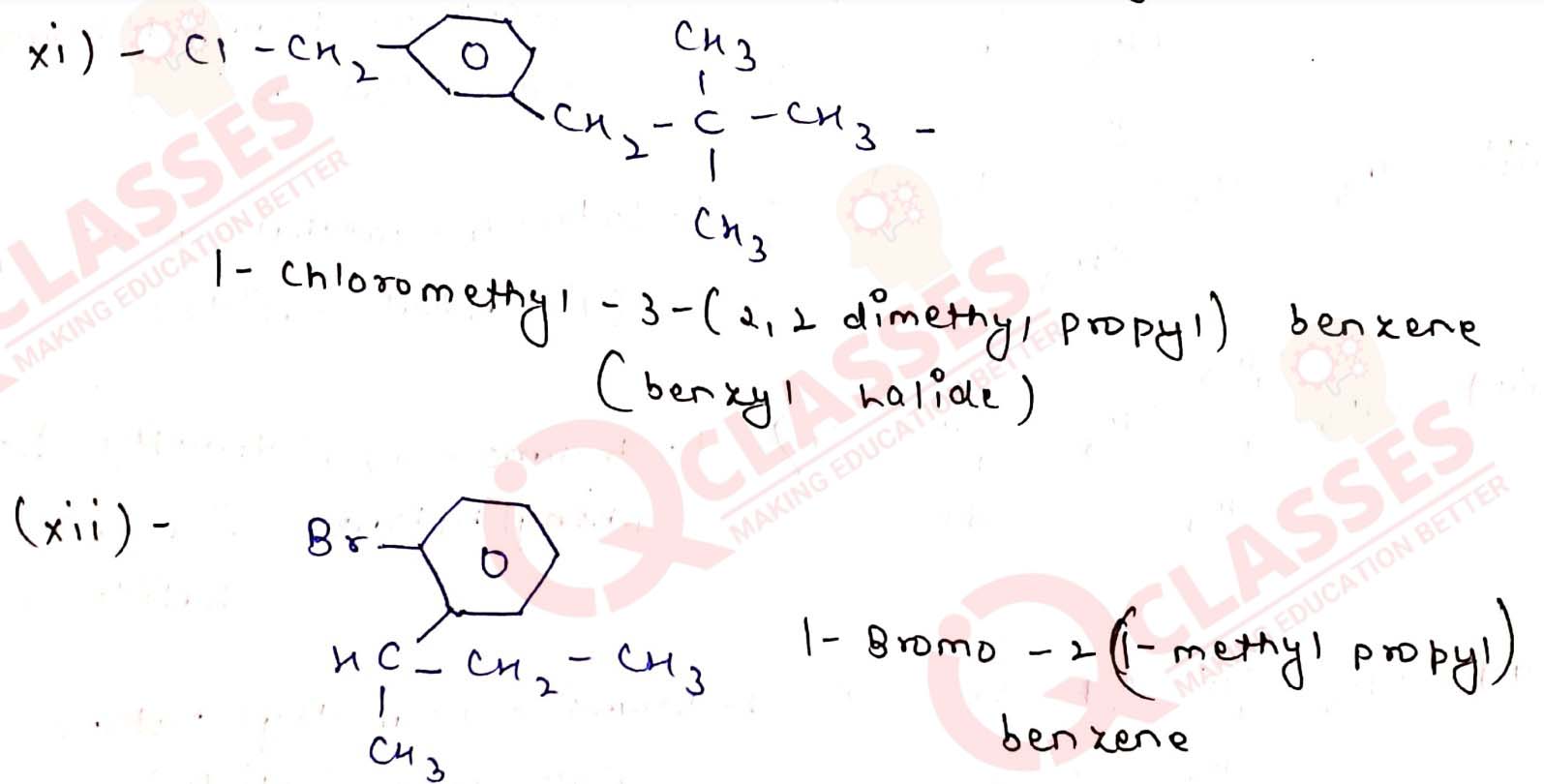
Name the following halides according to IUPAC system and classify them as
alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
 Solution
Solution
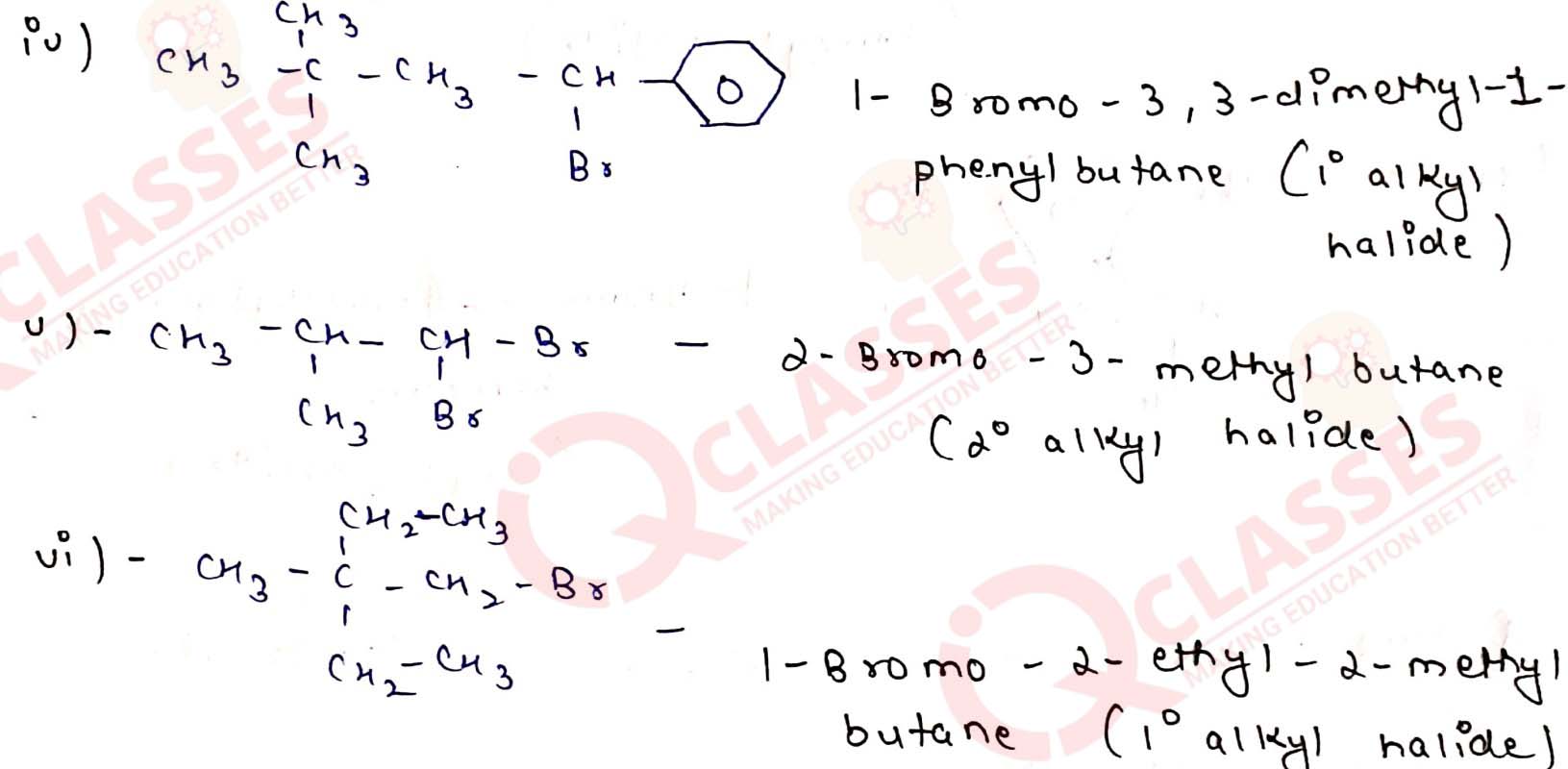

 Solution
Solution






Q10.2
Give the IUPAC names of the following compounds:
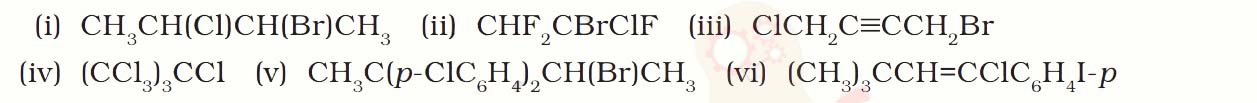 Solution
Solution
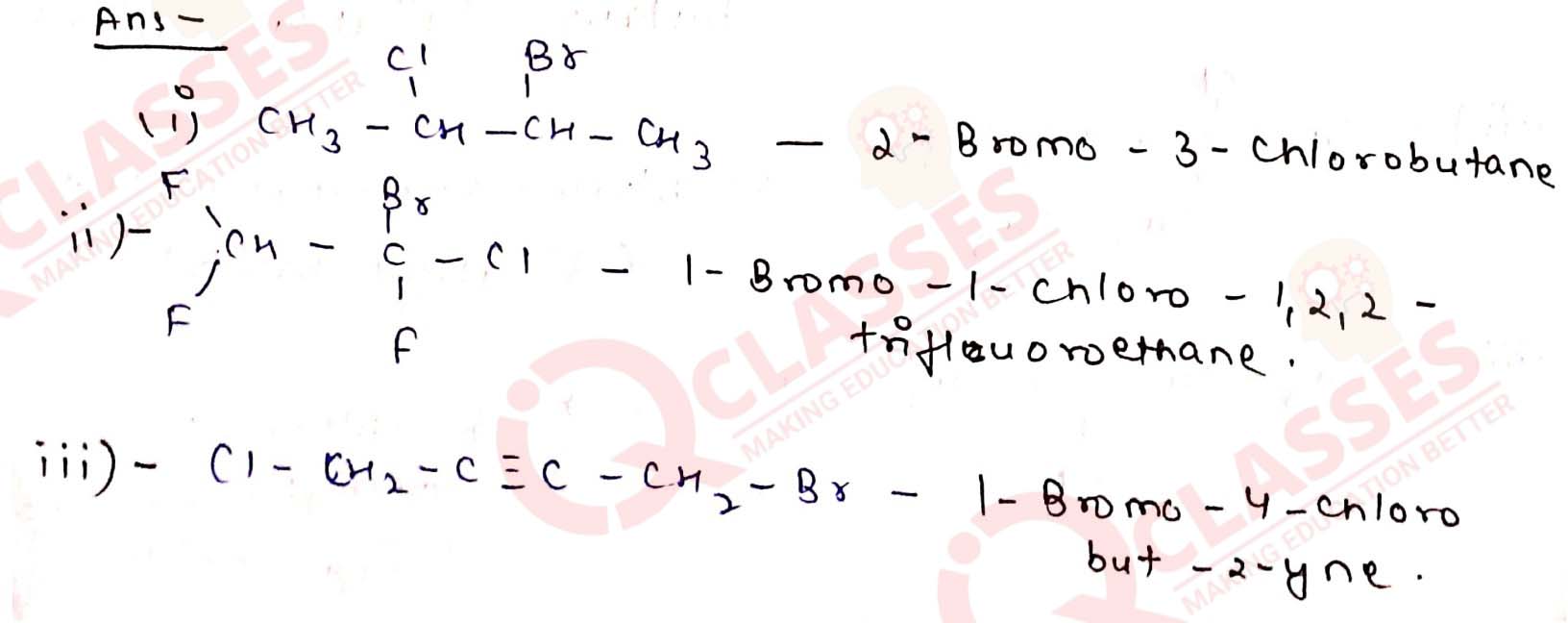
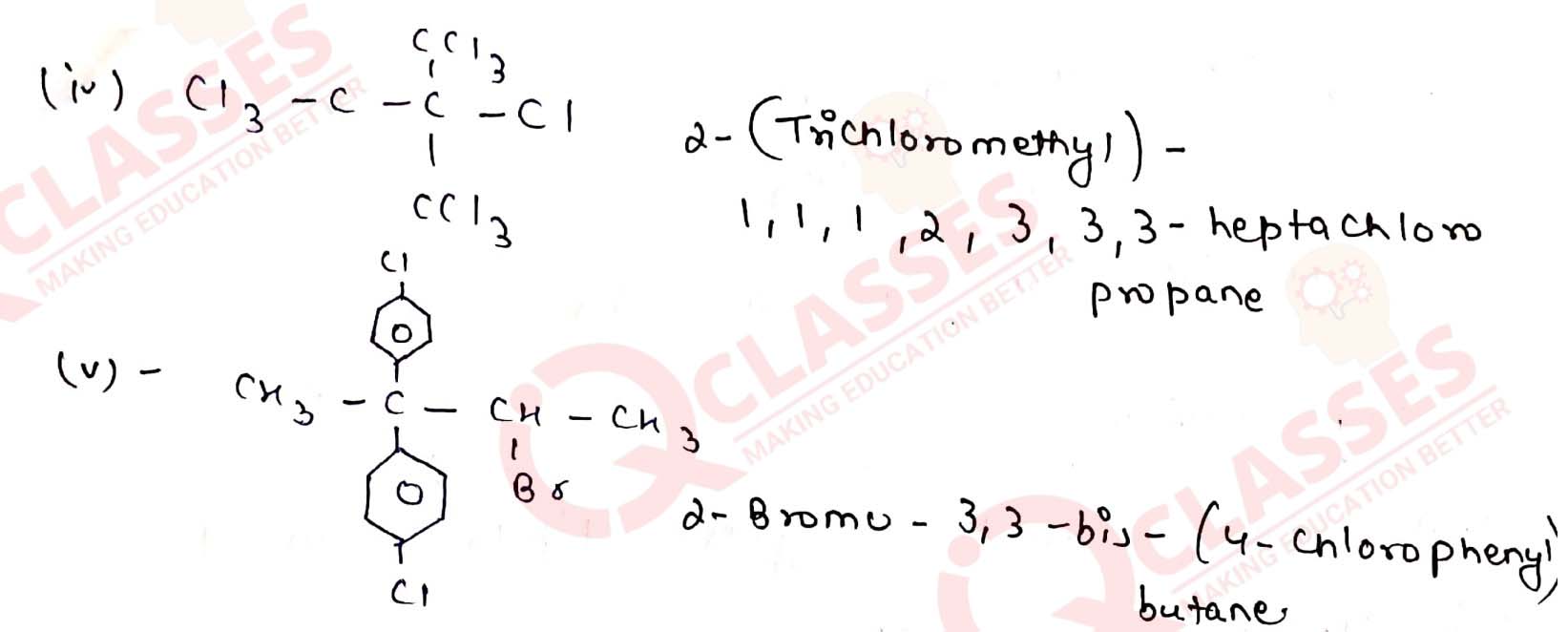
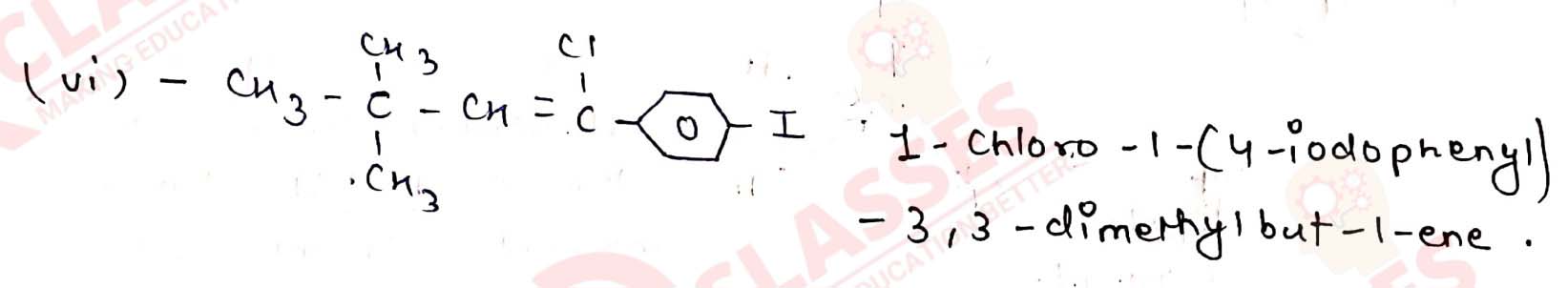
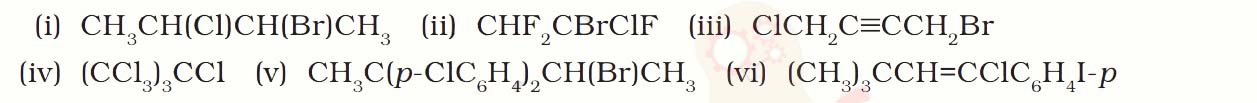 Solution
Solution



Q10.3
Write the structures of the following organic halogen compounds.
(i) 2-Chloro-3-methylpentane
(ii) p-Bromochlorobenzene
(iii) 1-Chloro-4-ethylcyclohexane
(iv) 2-(2-Chlorophenyl)-1-iodooctane
(v) 2-Bromobutane
(vi) 4-tert-Butyl-3-iodoheptane
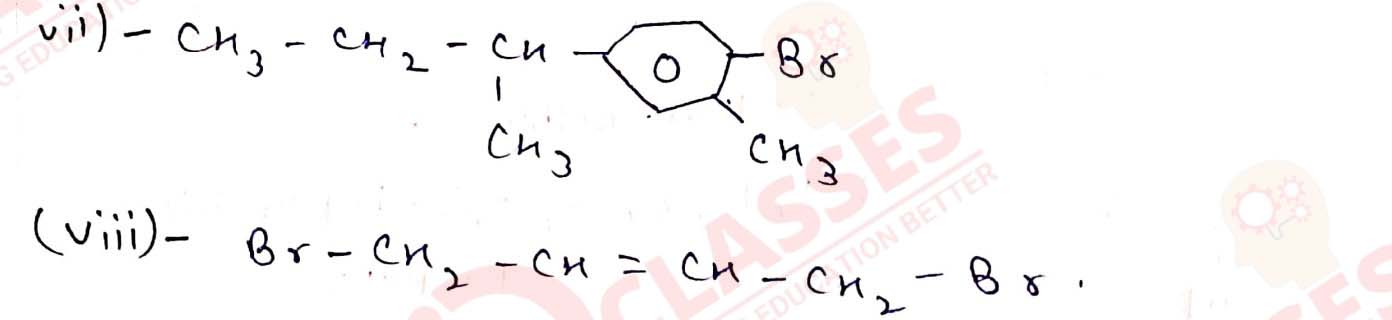
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
(viii) 1,4-Dibromobut-2-ene
Solution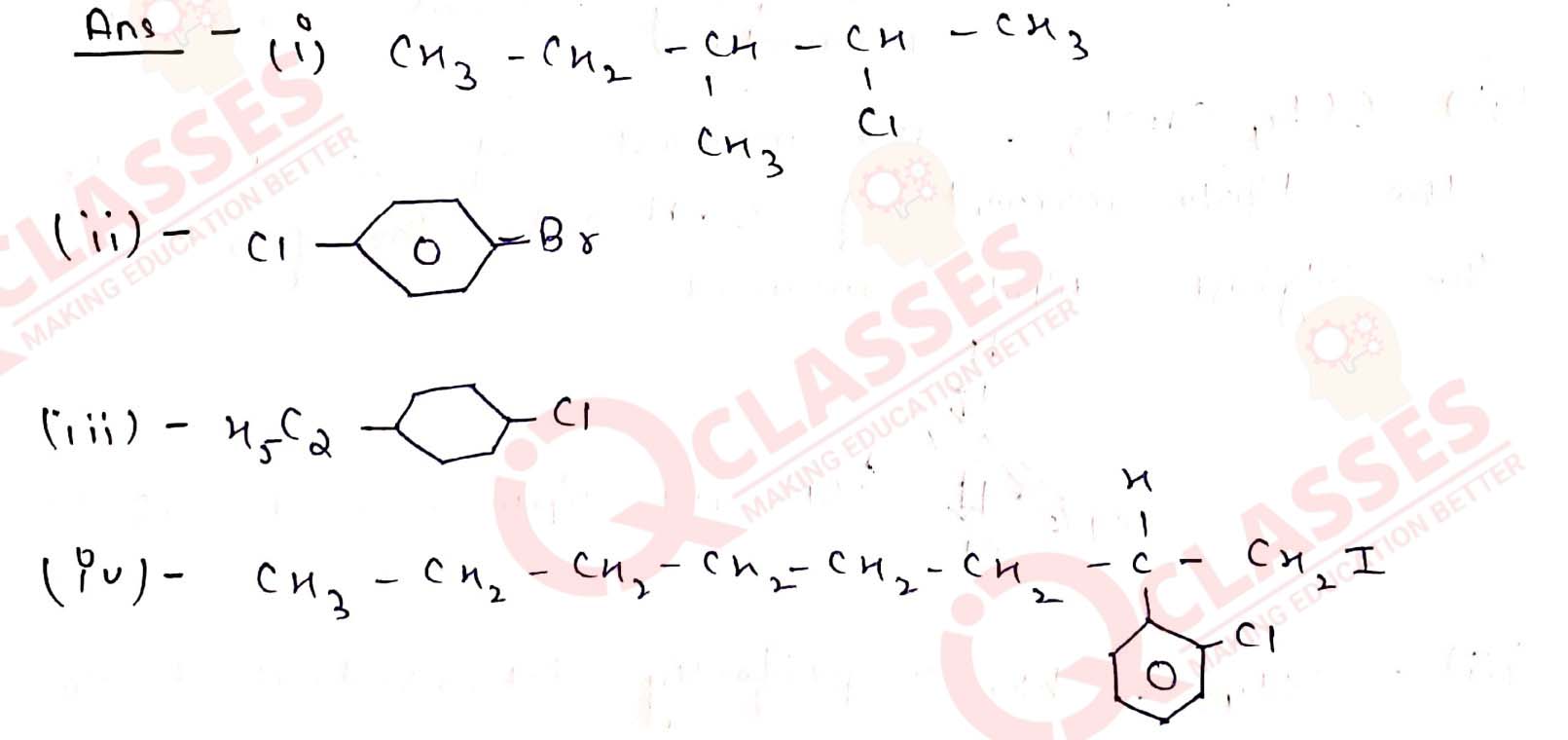
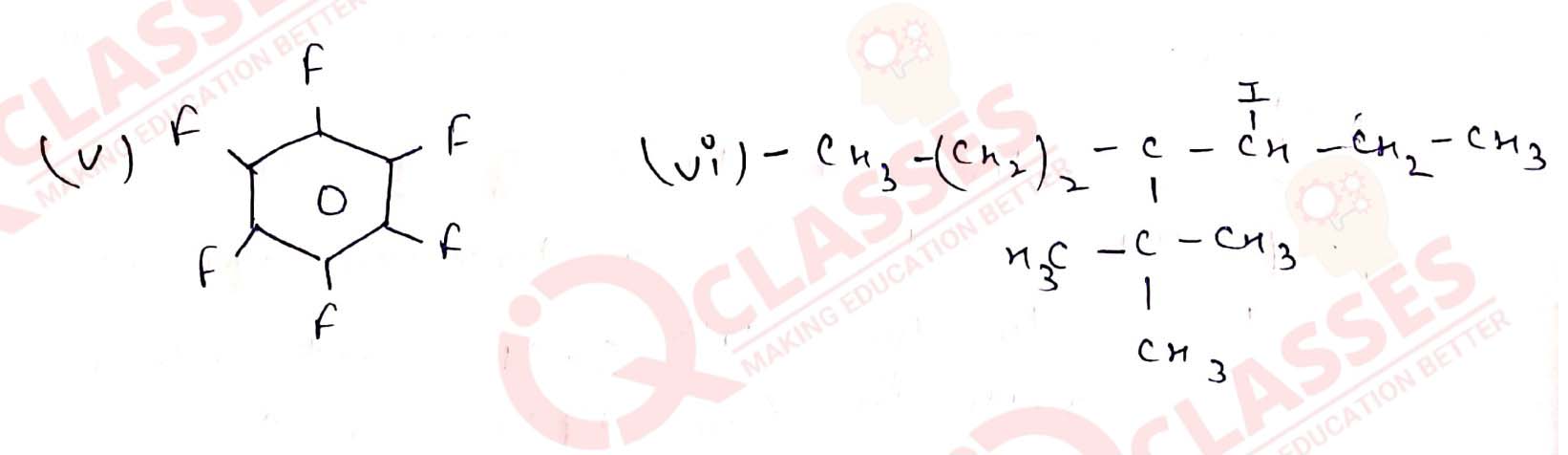
(i) 2-Chloro-3-methylpentane
(ii) p-Bromochlorobenzene
(iii) 1-Chloro-4-ethylcyclohexane
(iv) 2-(2-Chlorophenyl)-1-iodooctane
(v) 2-Bromobutane
(vi) 4-tert-Butyl-3-iodoheptane
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
(viii) 1,4-Dibromobut-2-ene
Solution

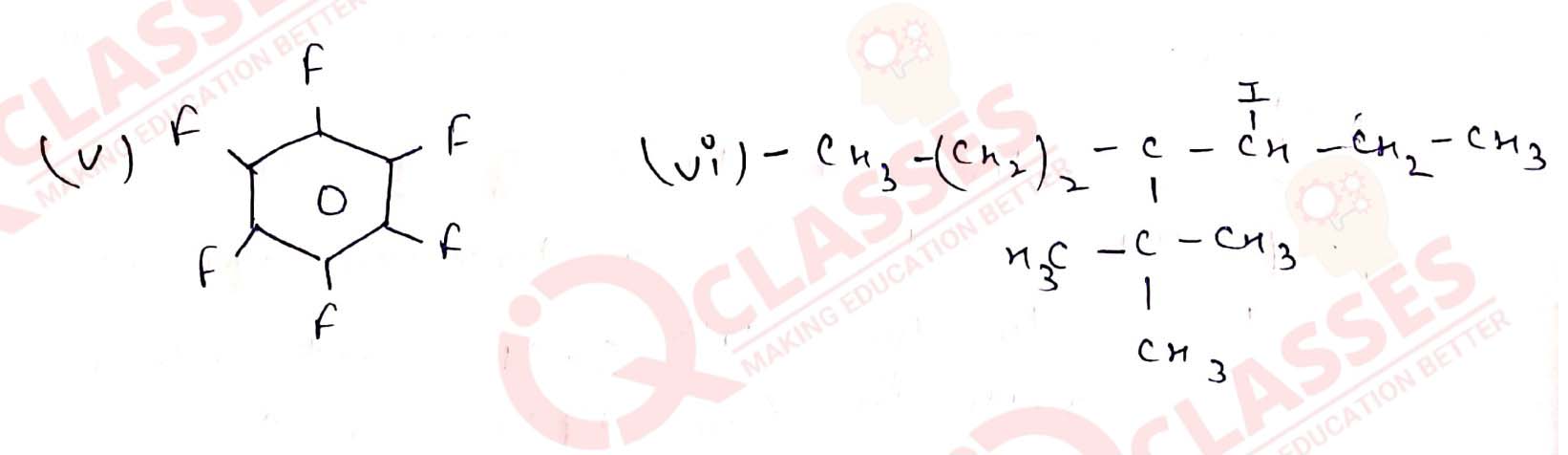

Q10.4
Which one of the following has the highest dipole moment?
(i) CH2Cl2
(ii) CHCl3
(iii) CCl4 Solution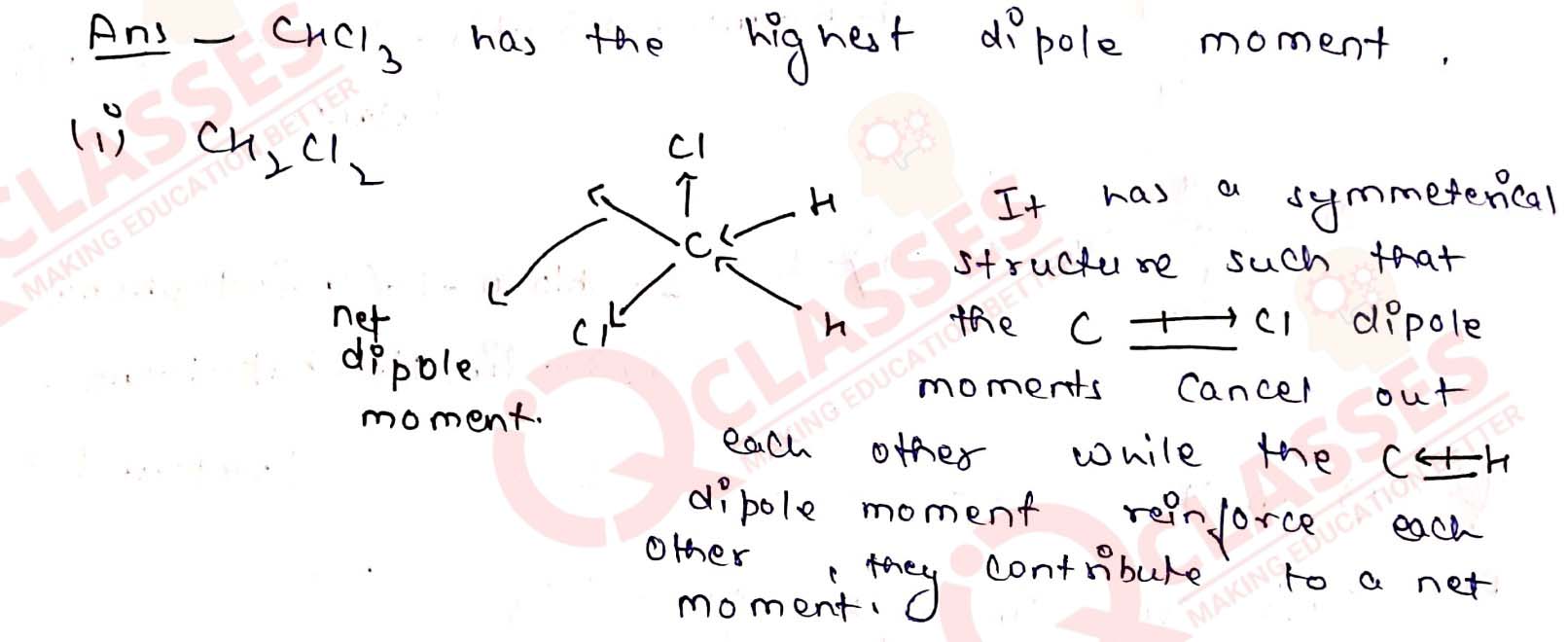
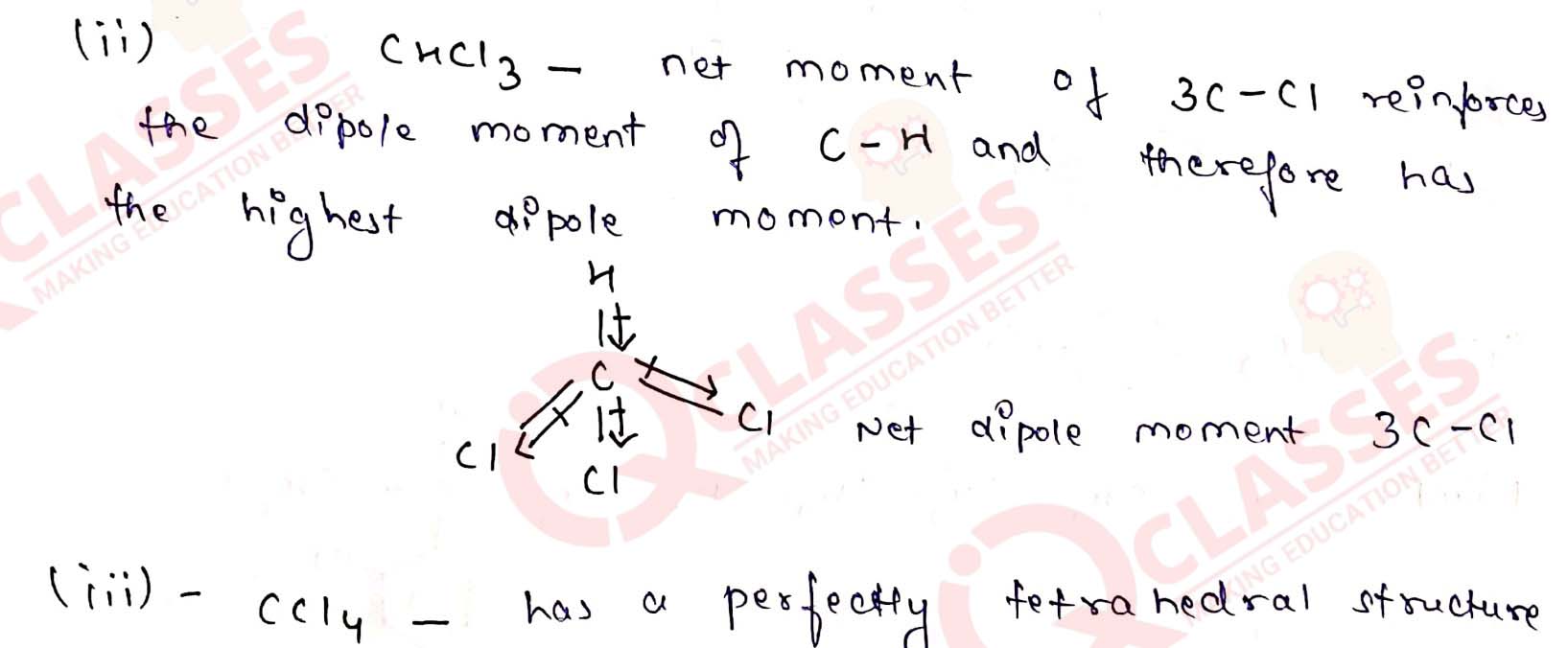

(i) CH2Cl2
(ii) CHCl3
(iii) CCl4 Solution



Q10.5
A hydrocarbon C5H10 does not react with chlorine in dark but gives a single
monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon
Solution




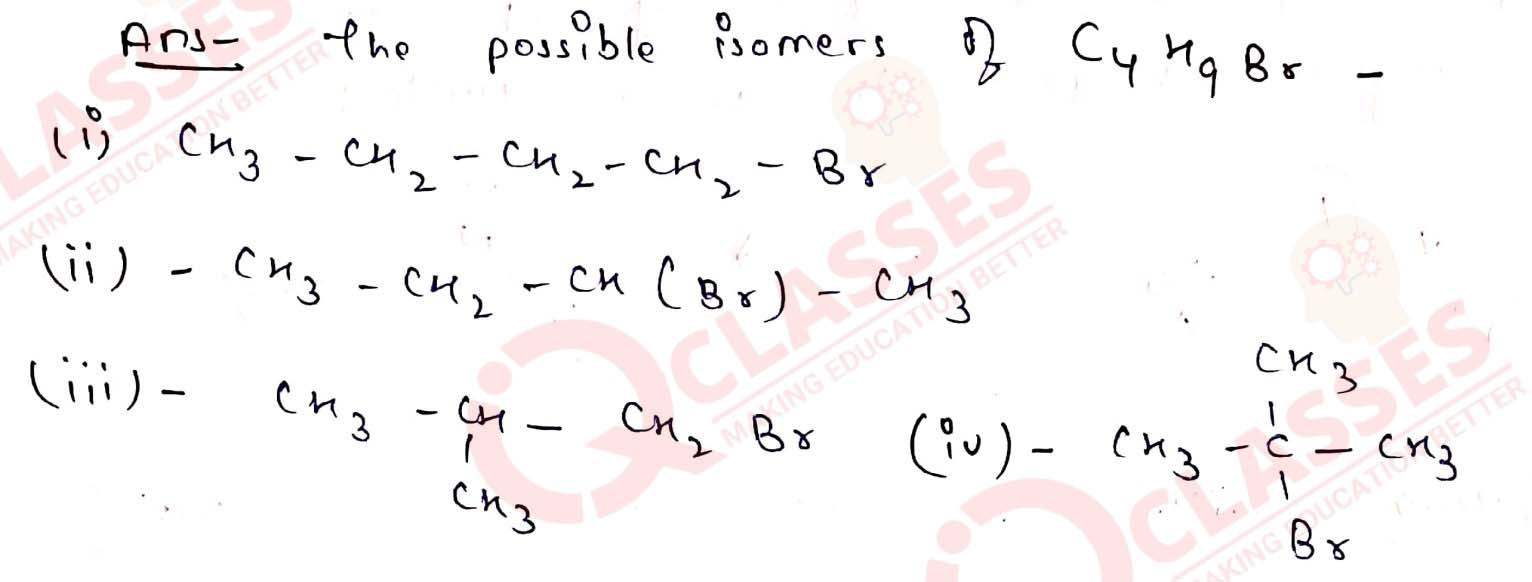
Q10.6
Write the isomers of the compound having formula C4H9Br
Solution

Q10.7
Write the equations for the preparation of 1-iodobutane from
(i) 1-butanol (ii) 1-chlorobutane (iii) but-1-ene. Solution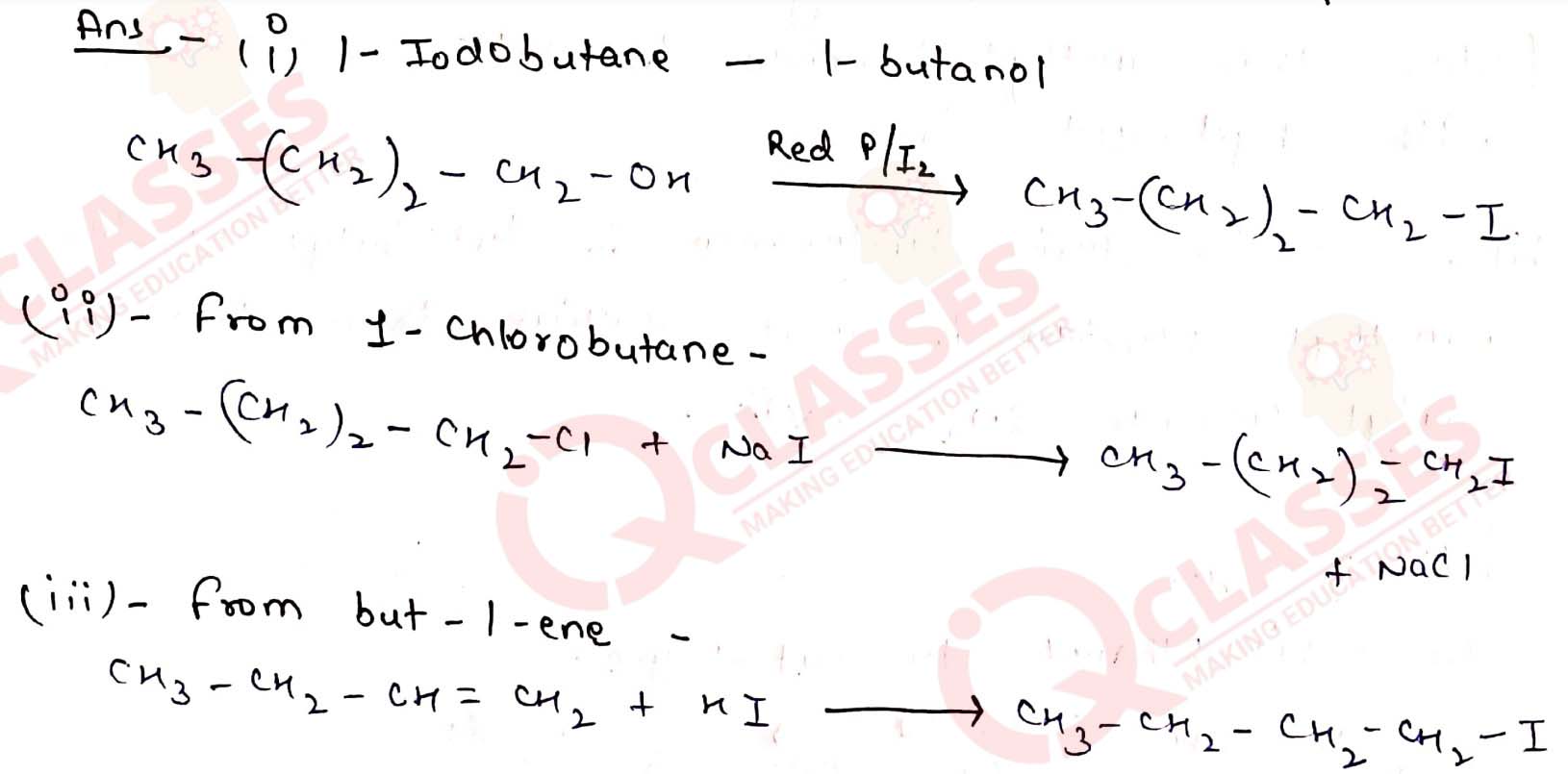
(i) 1-butanol (ii) 1-chlorobutane (iii) but-1-ene. Solution

Q10.8
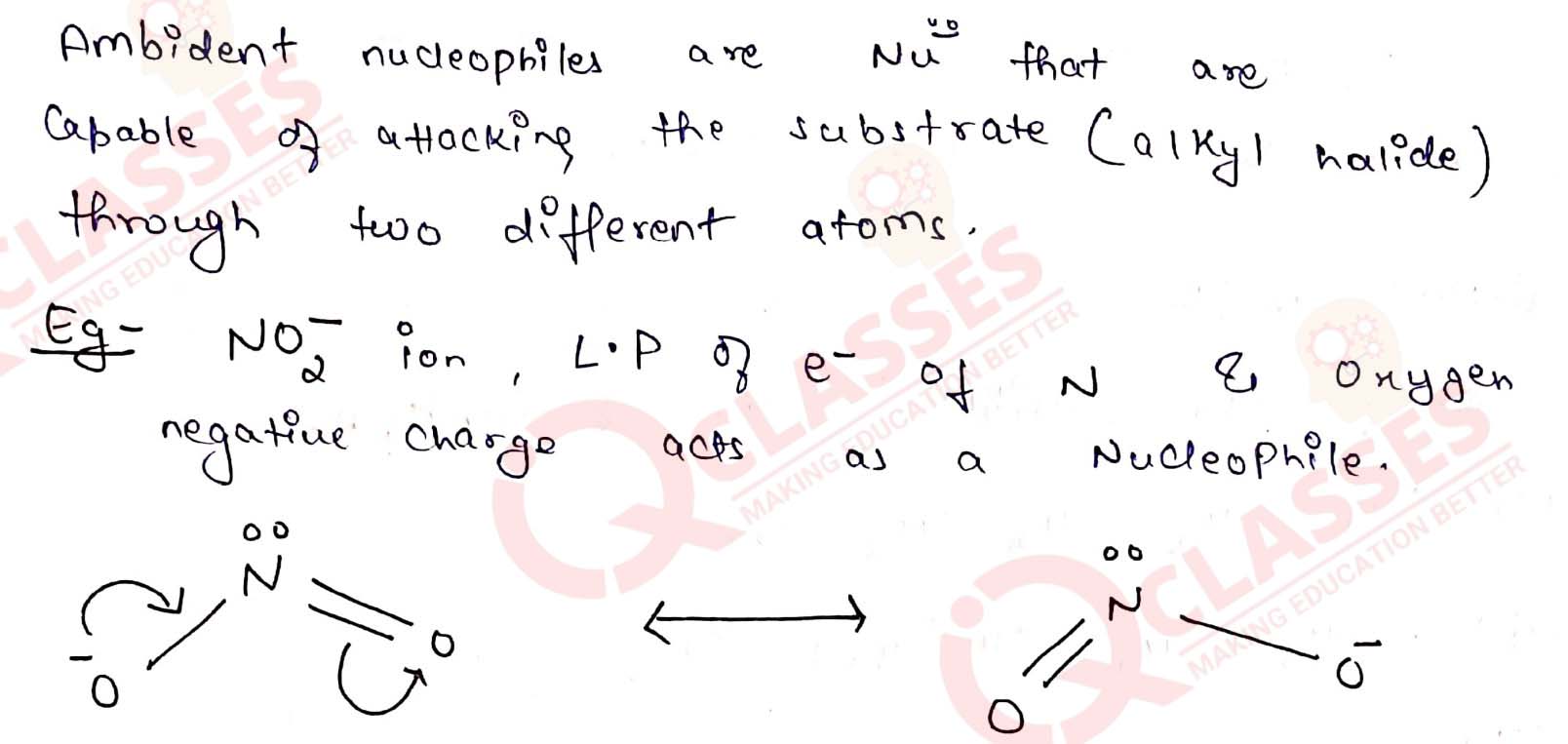
What are ambident nucleophiles? Explain with an example.
Solution

Q10.9
Which compound in each of the following pairs will react faster in SN
2 reaction
with –OH?
(i) CH3Br or CH3 I
(ii) (CH3 ) 3CCl or CH3Cl
Solution
(i) CH3Br or CH3 I
(ii) (CH3 ) 3CCl or CH3Cl
Solution

Q10.10
Predict all the alkenes that would be formed by dehydrohalogenation of the
following halides with sodium ethoxide in ethanol and identify the major alkene:
(i) 1-Bromo-1-methylcyclohexane
(ii) 2-Chloro-2-methylbutane
(iii) 2,2,3-Trimethyl-3-bromopentane.
Solution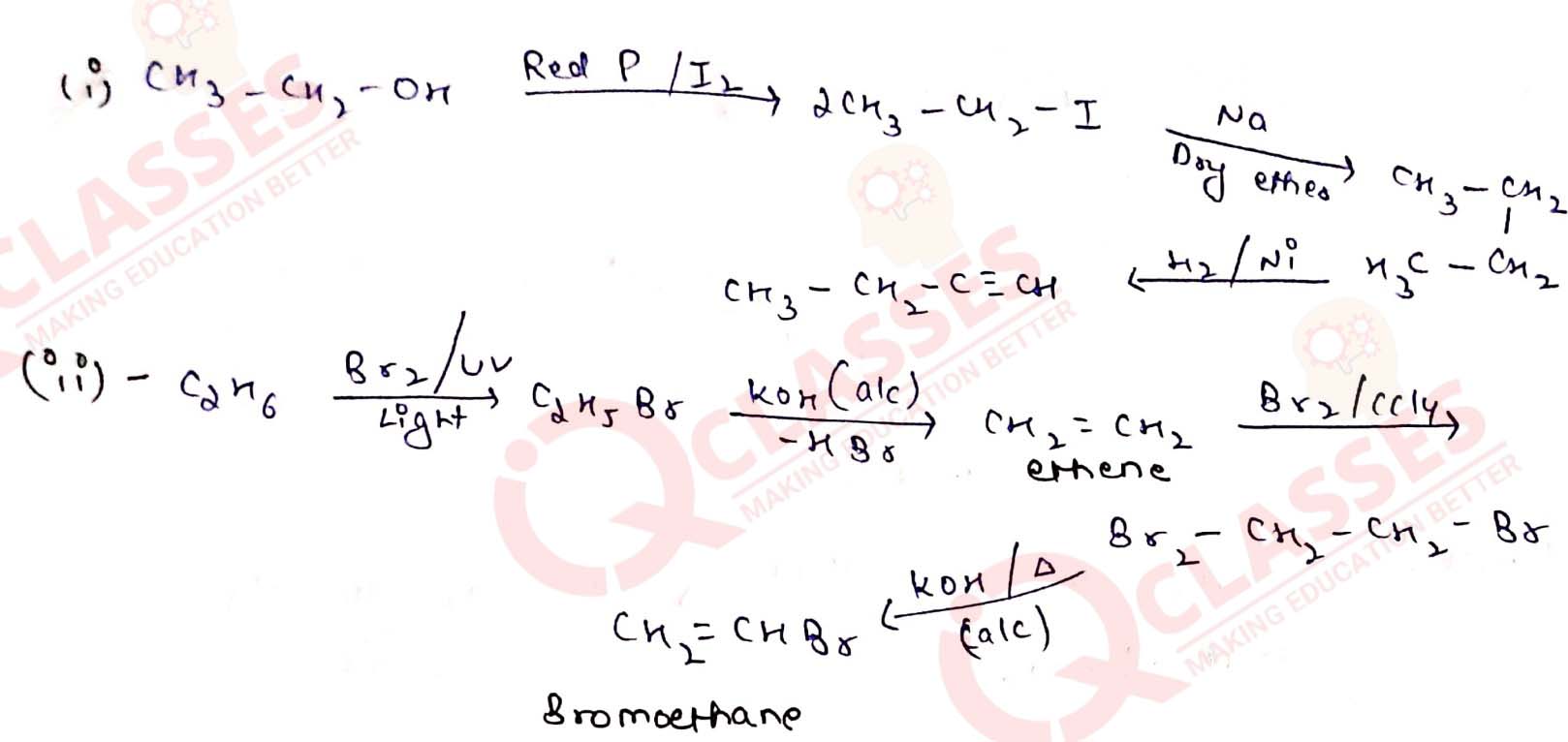
(i) 1-Bromo-1-methylcyclohexane
(ii) 2-Chloro-2-methylbutane
(iii) 2,2,3-Trimethyl-3-bromopentane.
Solution





Q10.11
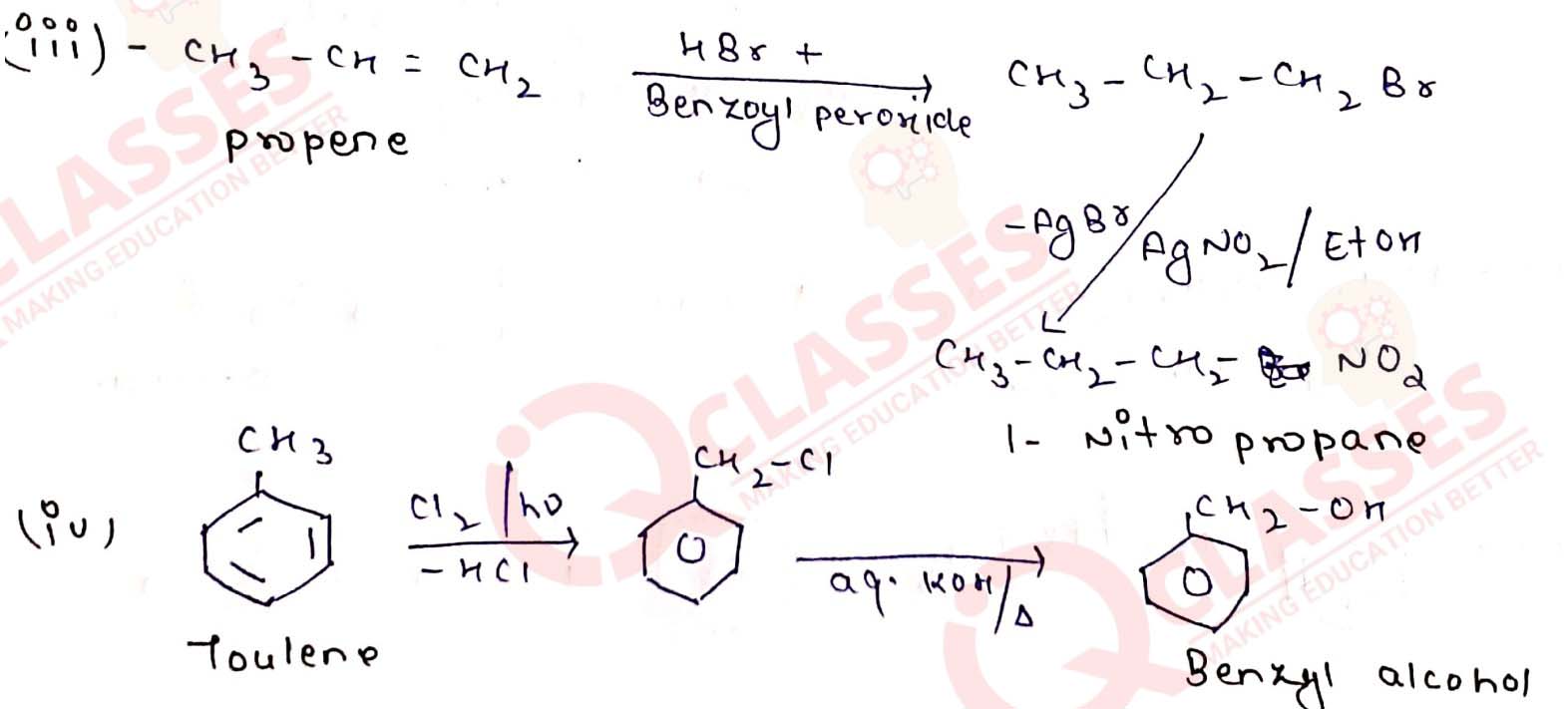
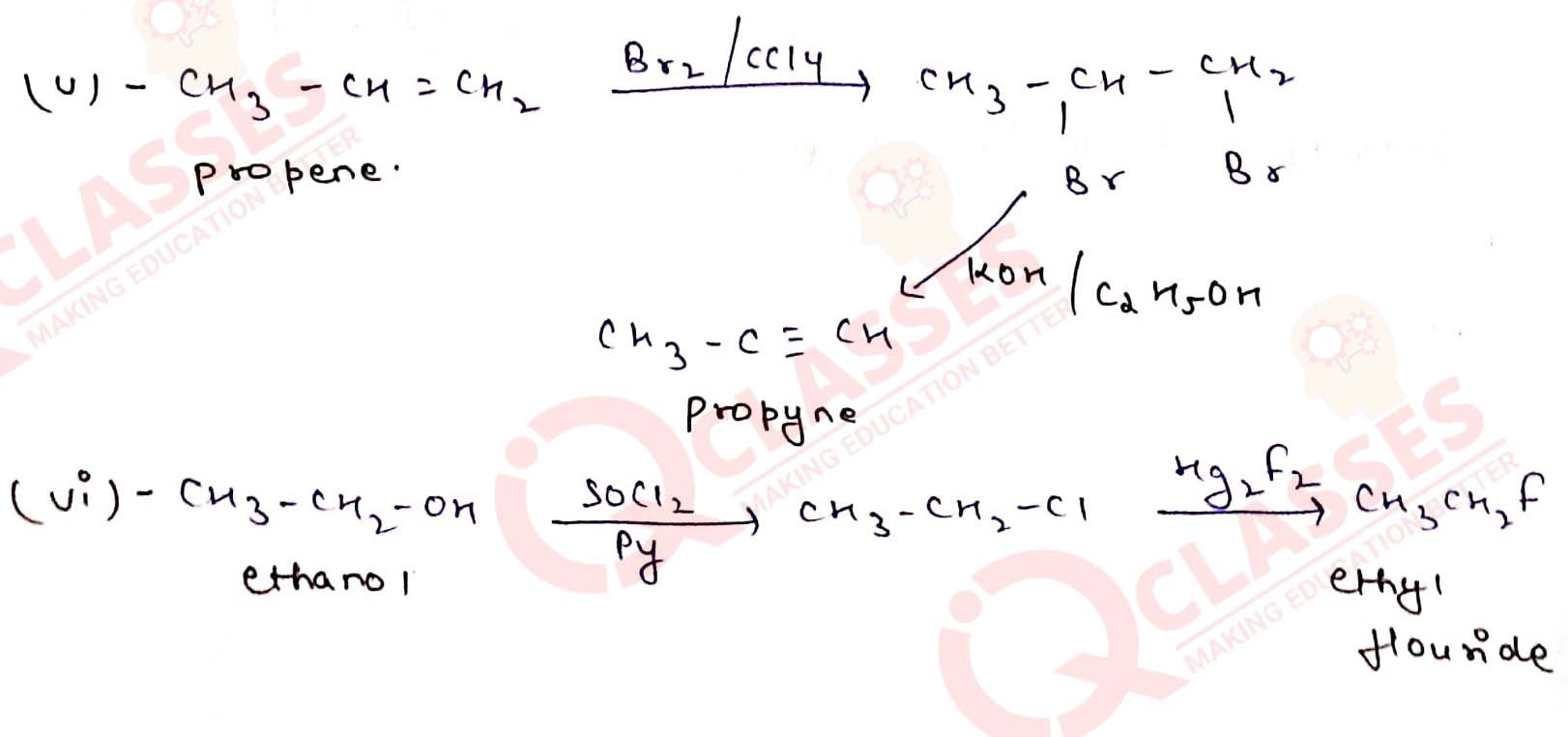
How will you bring about the following conversions?
(i) Ethanol to but-1-yne
(ii) Ethane to bromoethene
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
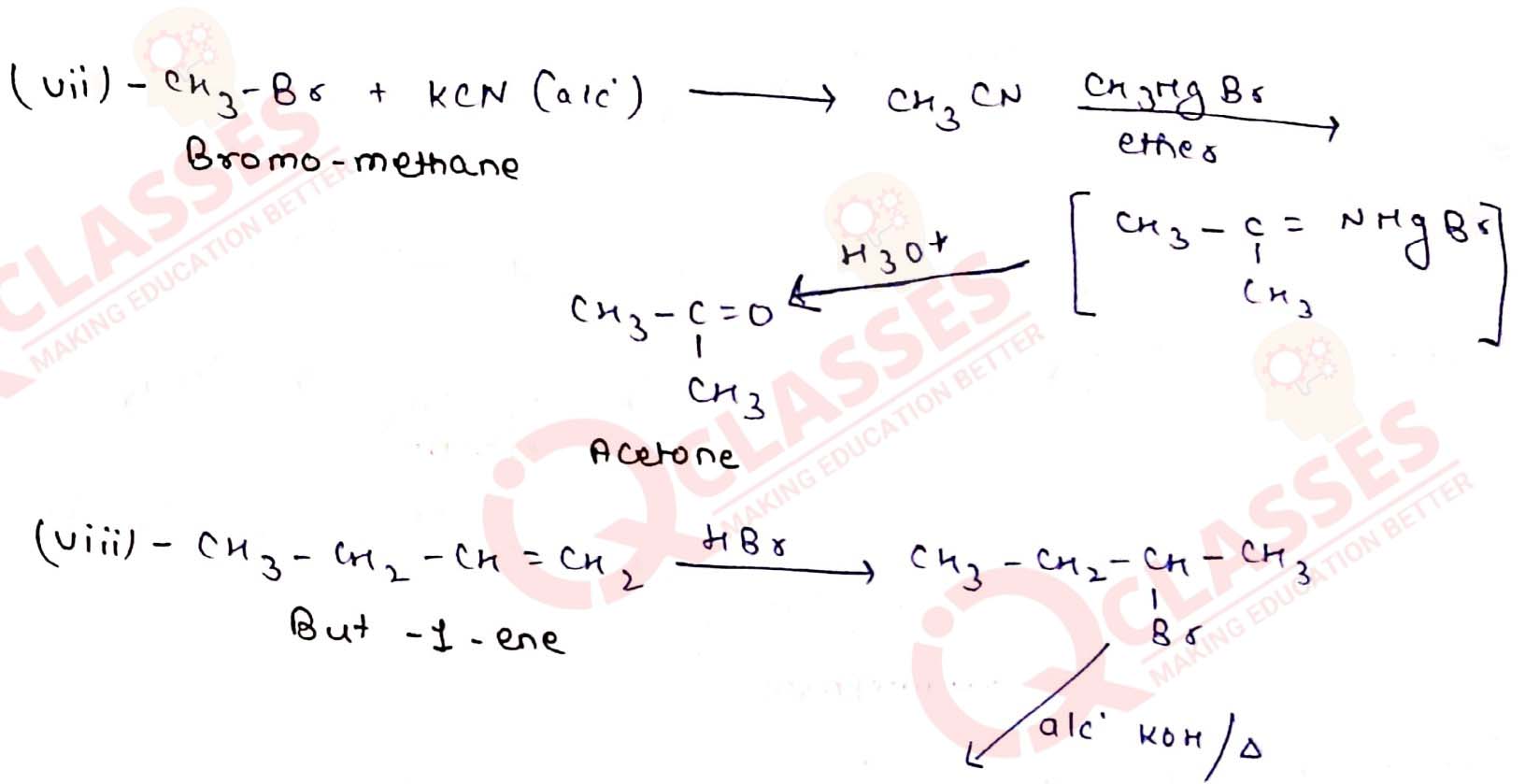
(vii) Bromomethane to propanone
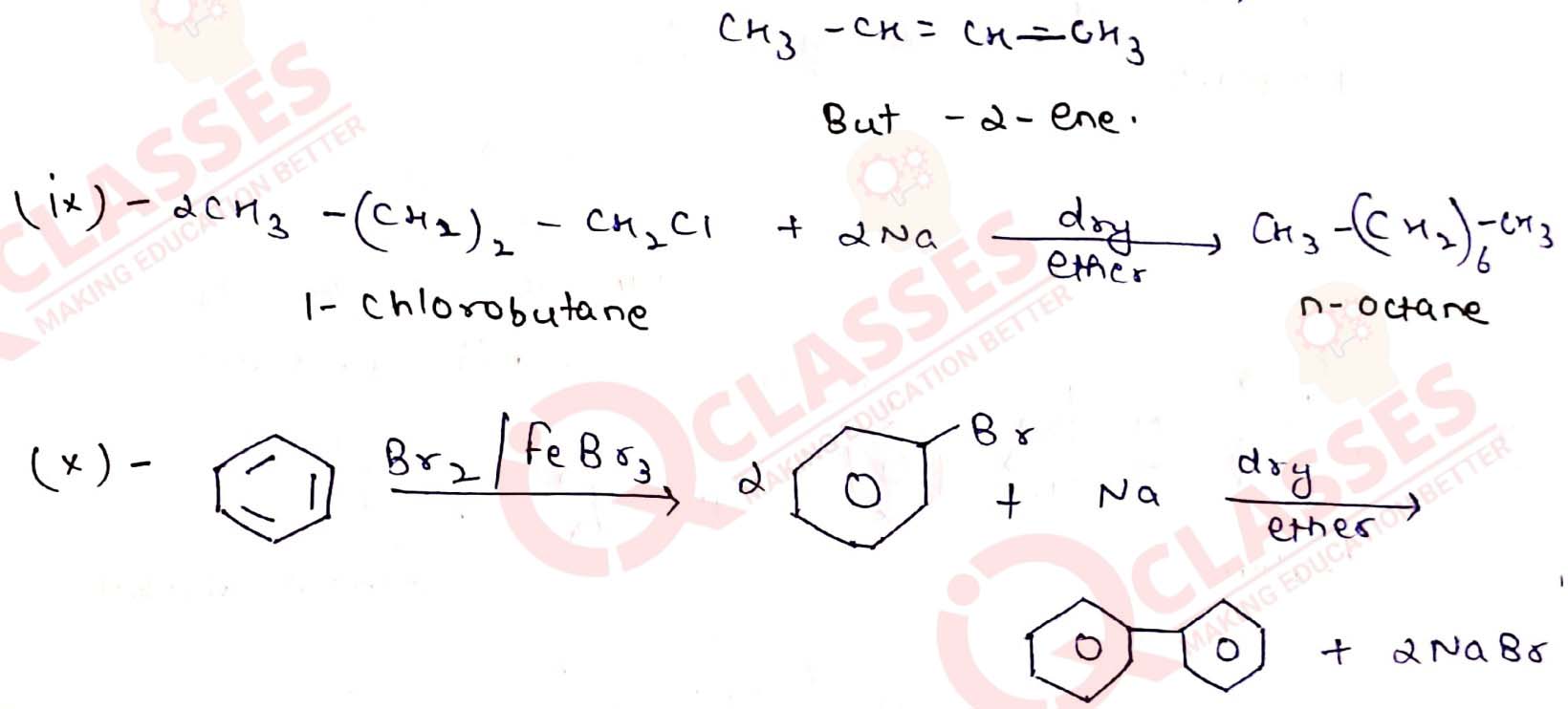
(viii) But-1-ene to but-2-ene
(ix) 1-Chlorobutane to n-octane
(x) Benzene to biphenyl.
Solution
(i) Ethanol to but-1-yne
(ii) Ethane to bromoethene
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
(vii) Bromomethane to propanone
(viii) But-1-ene to but-2-ene
(ix) 1-Chlorobutane to n-octane
(x) Benzene to biphenyl.
Solution
Q10.12
Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides, though polar, are immiscible with water?
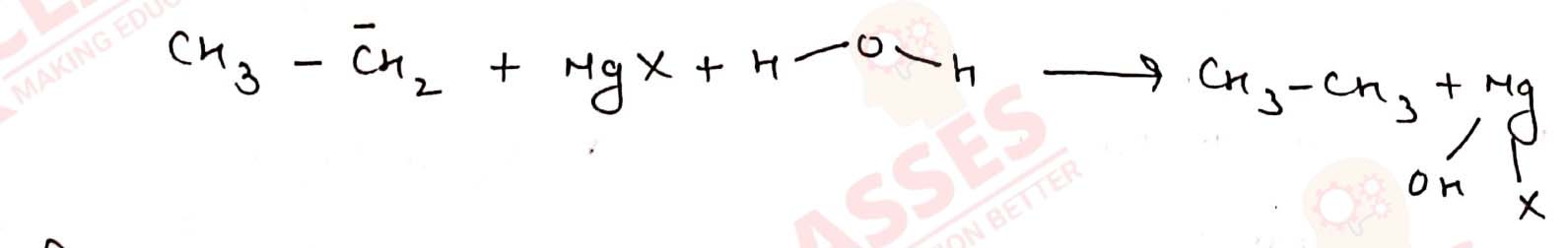
(iii) Grignard reagents should be prepared under anhydrous conditions?
Solution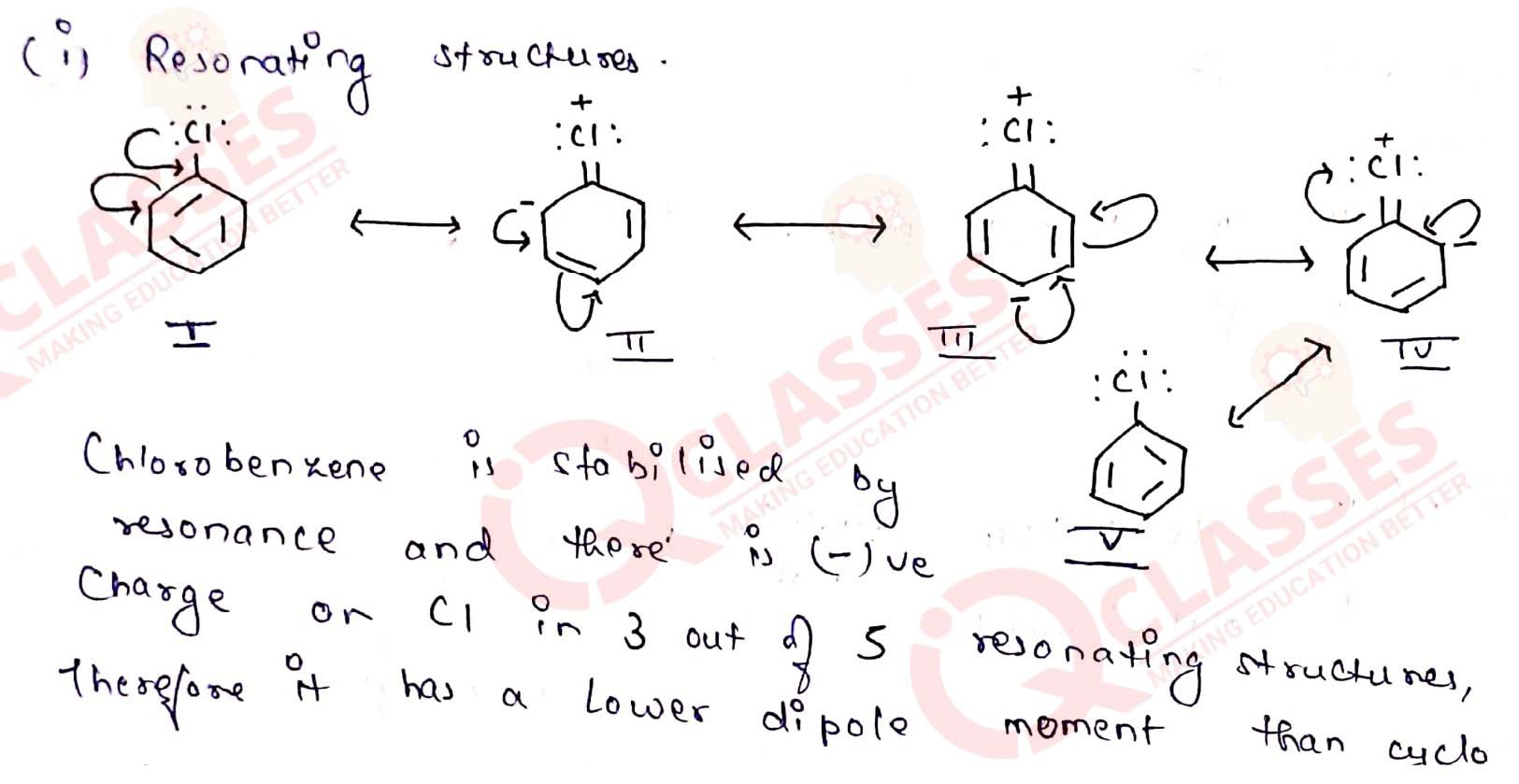

(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
Solution



Q10.13
Give the uses of freon 12, DDT, carbon tetrachloride and iodoform
Solution
Q10.14
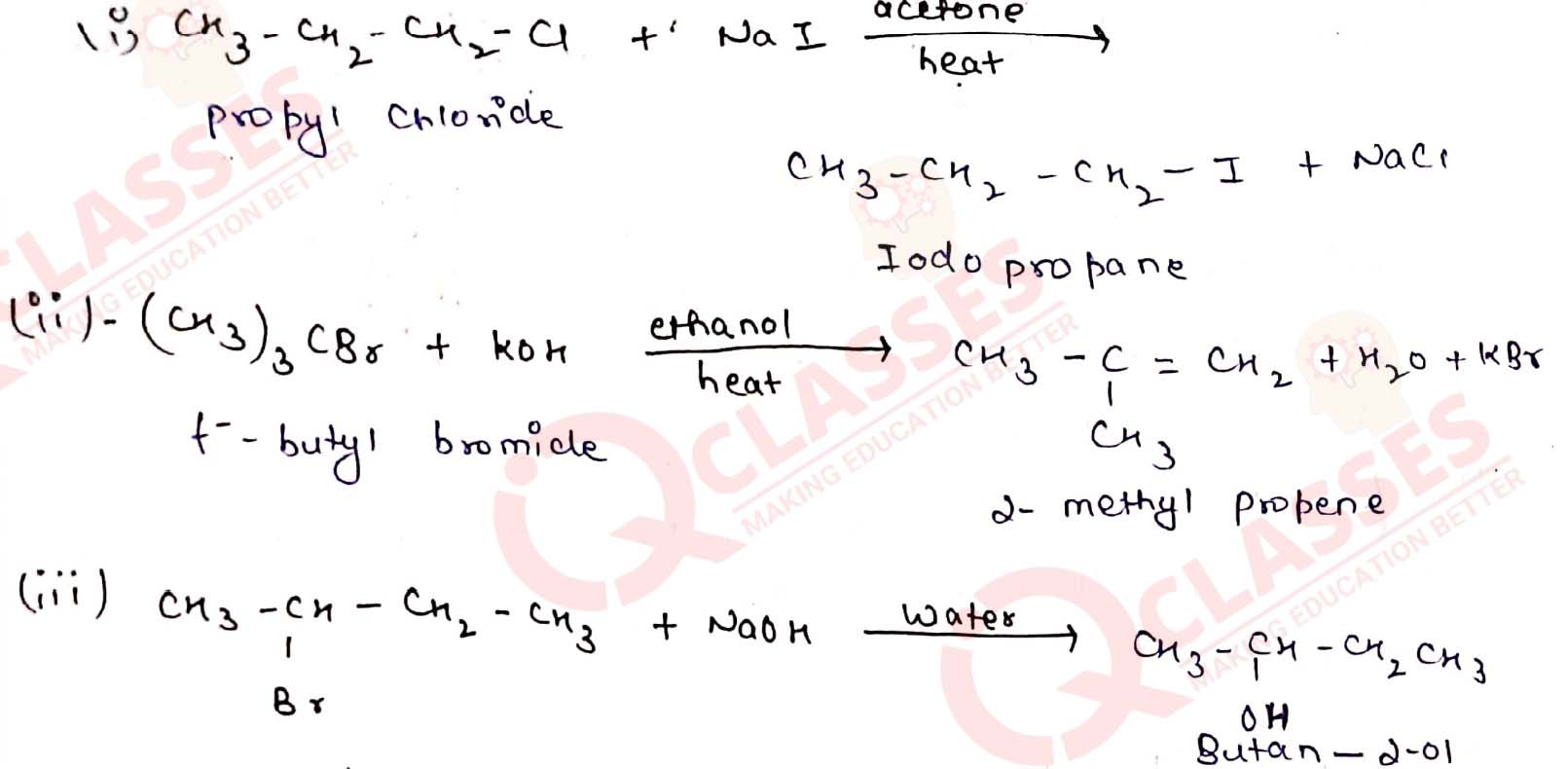
Write the structure of the major organic product in each of the following reactions:
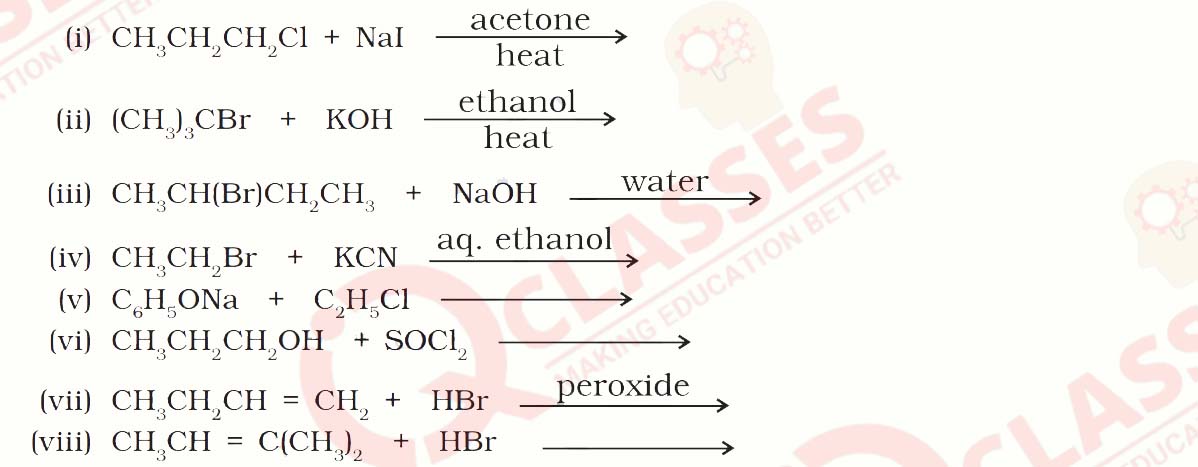 Solution
Solution
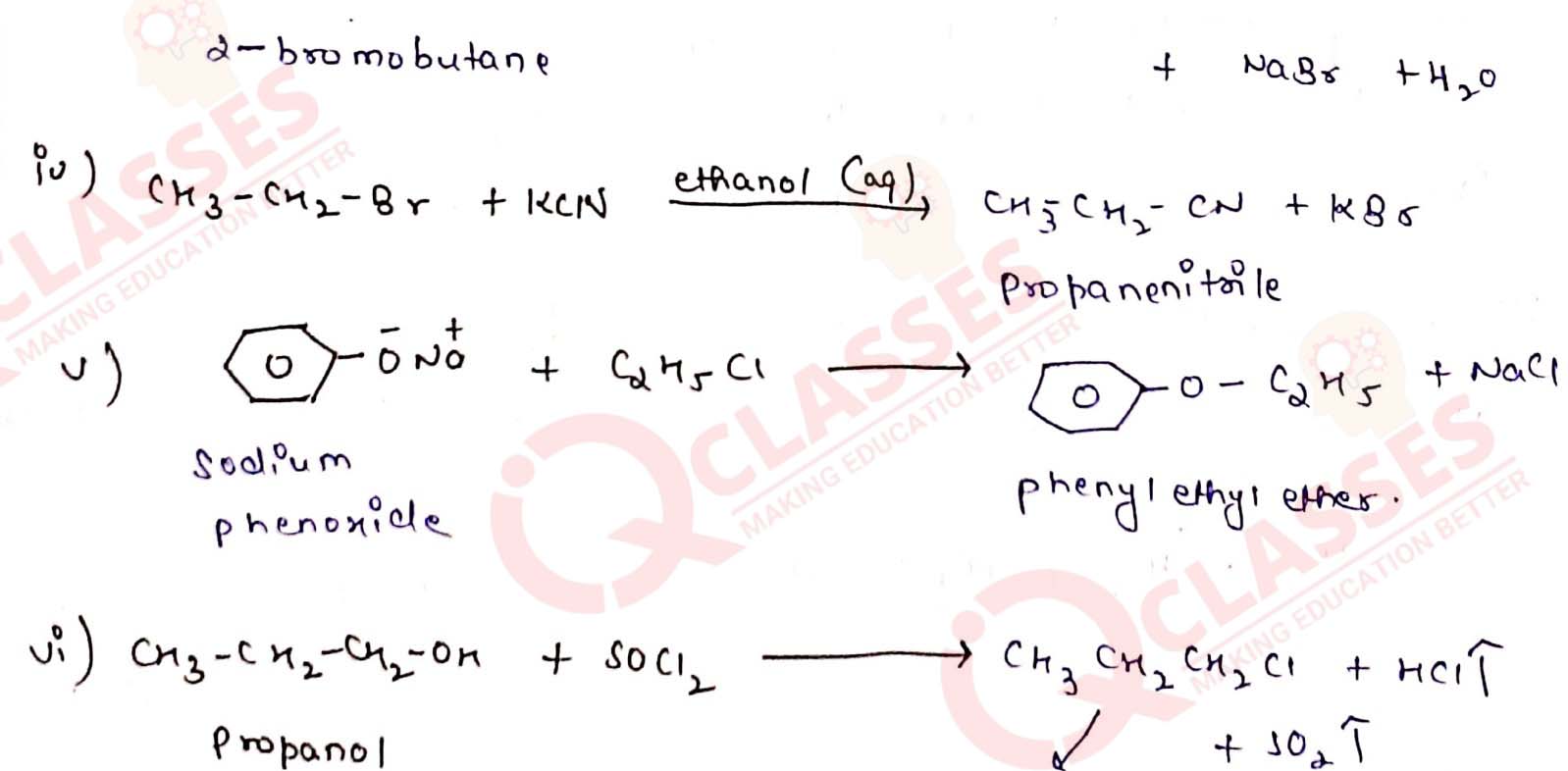
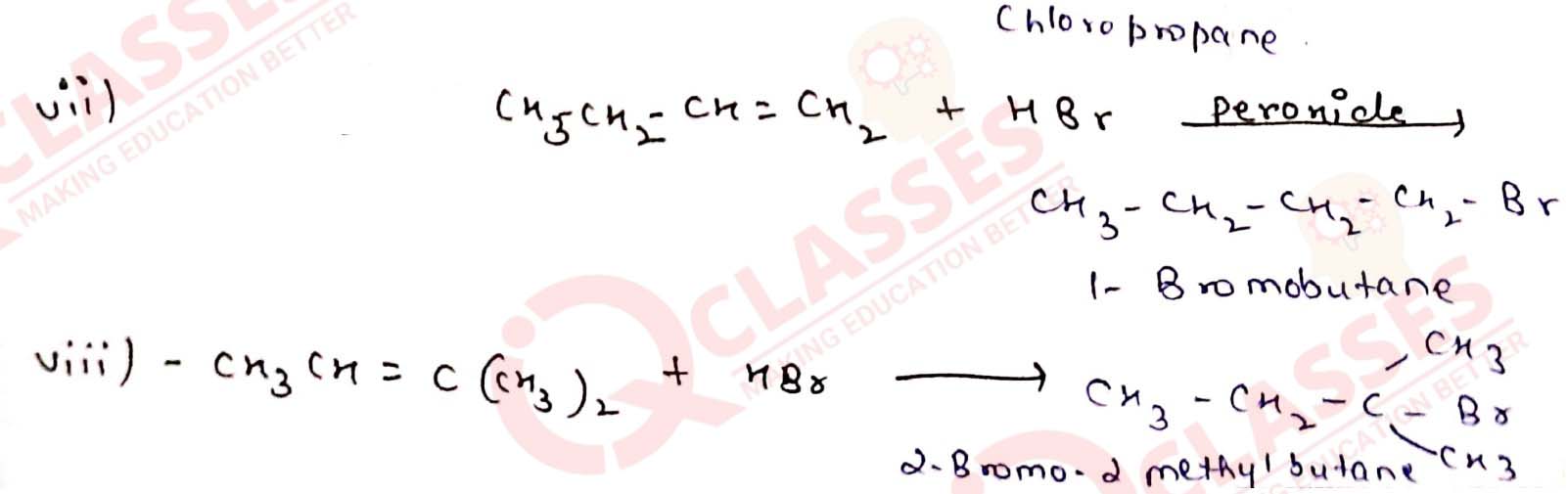
 Solution
Solution



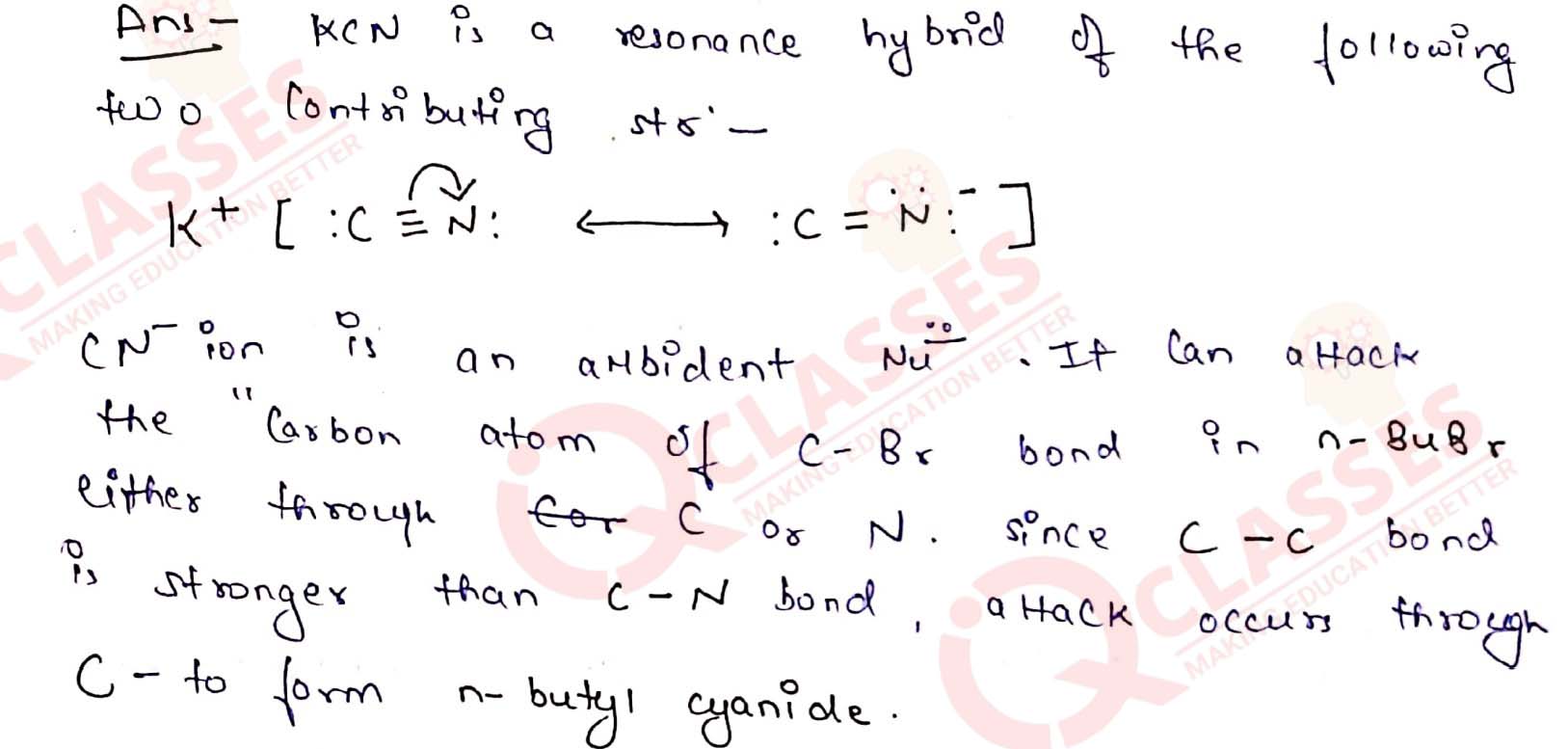
Q10.15
Write the mechanism of the following reaction:
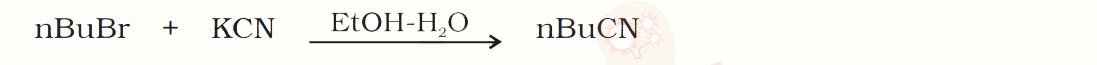 Solution
Solution
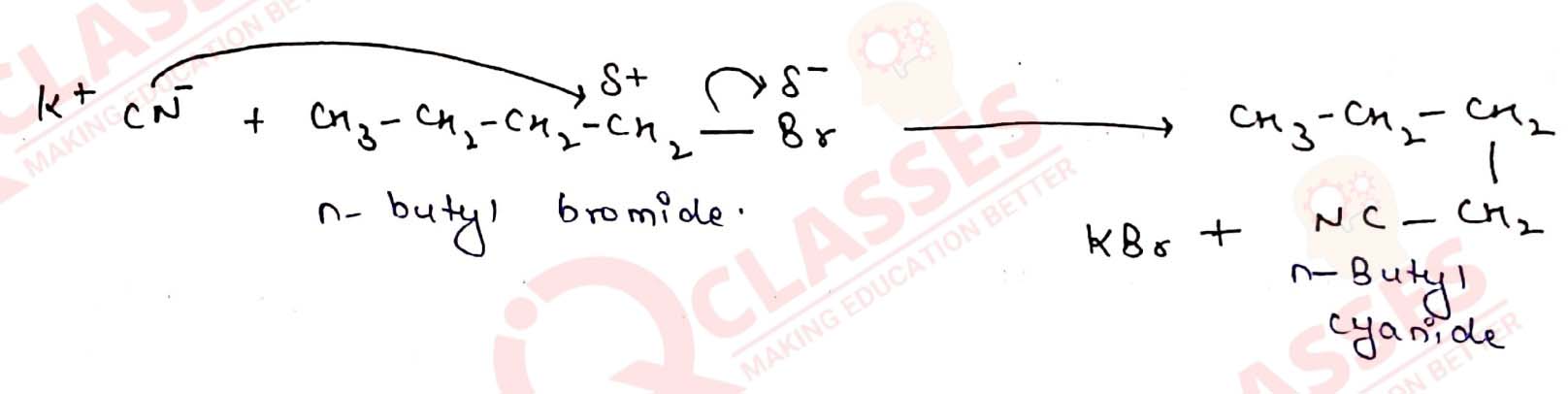
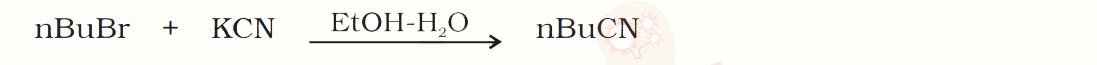 Solution
Solution

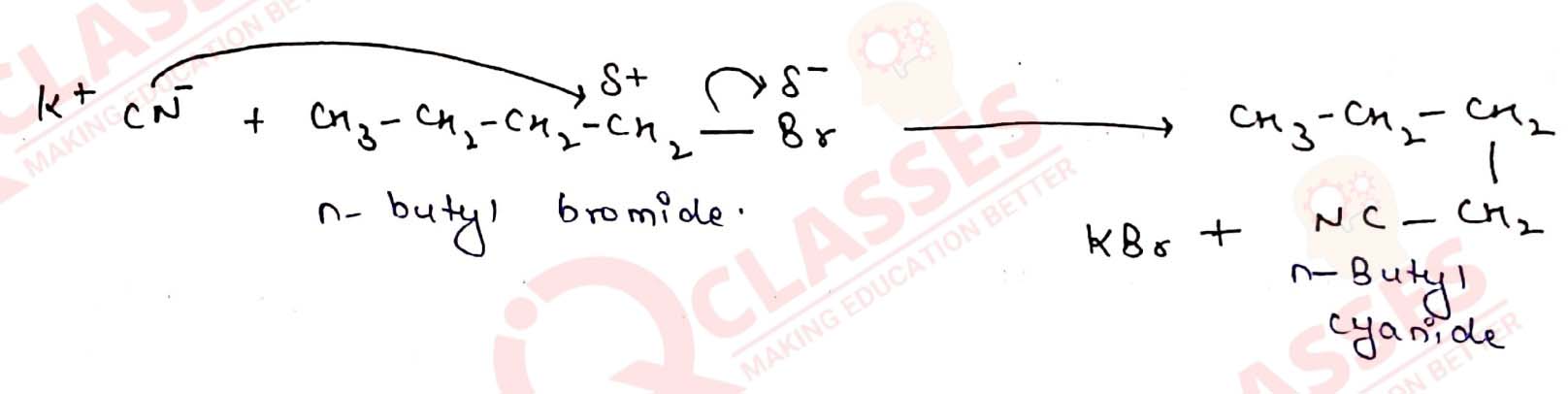
Q10.16
Arrange the compounds of each set in order of reactivity towards SN2
displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
(iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane.
Solution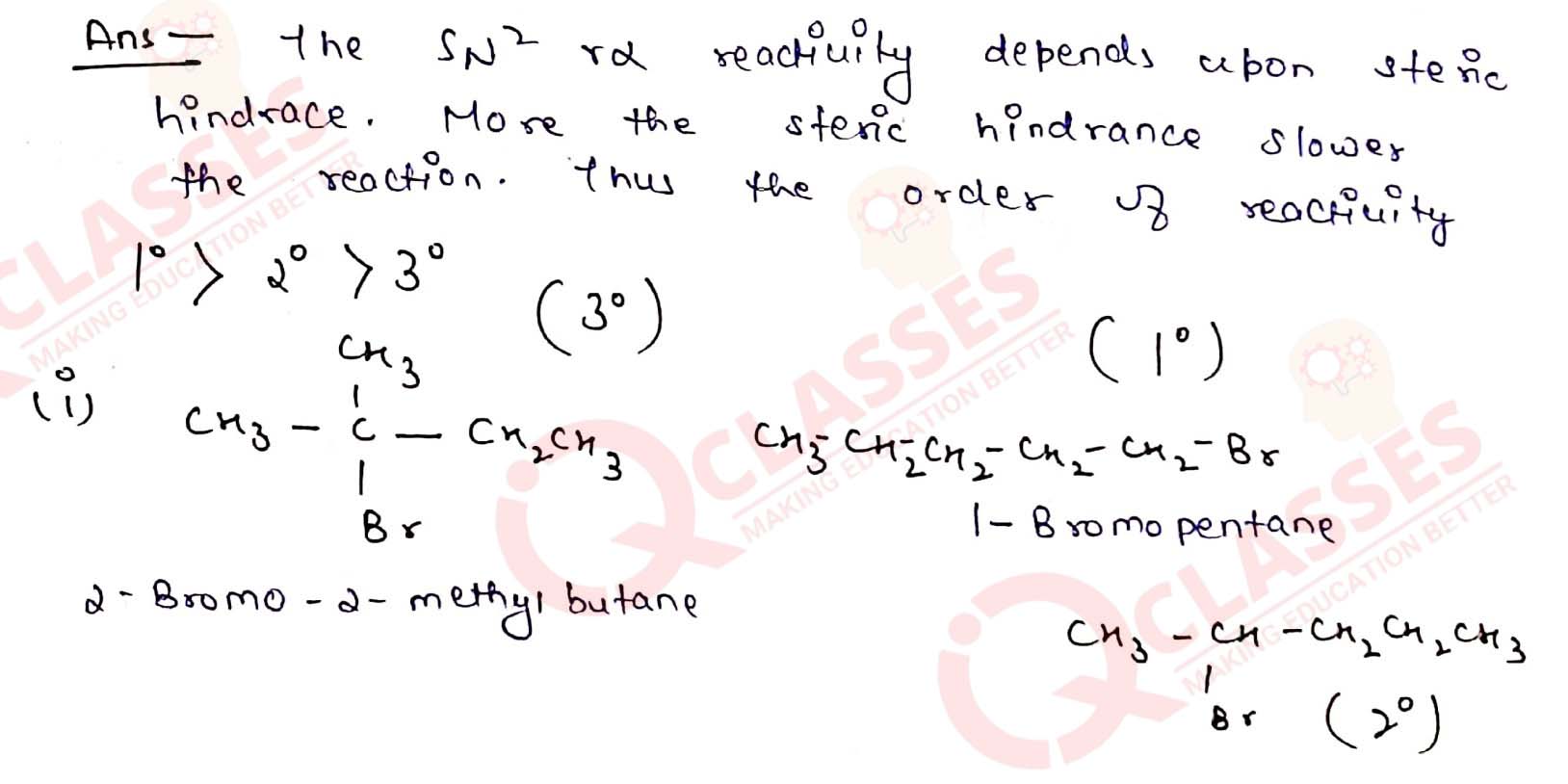
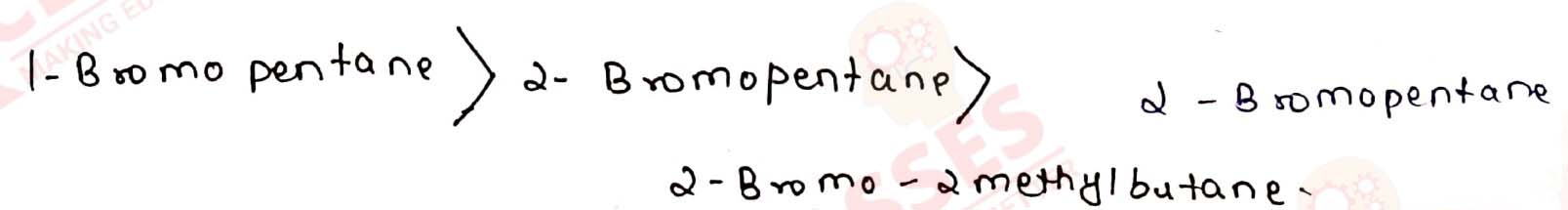
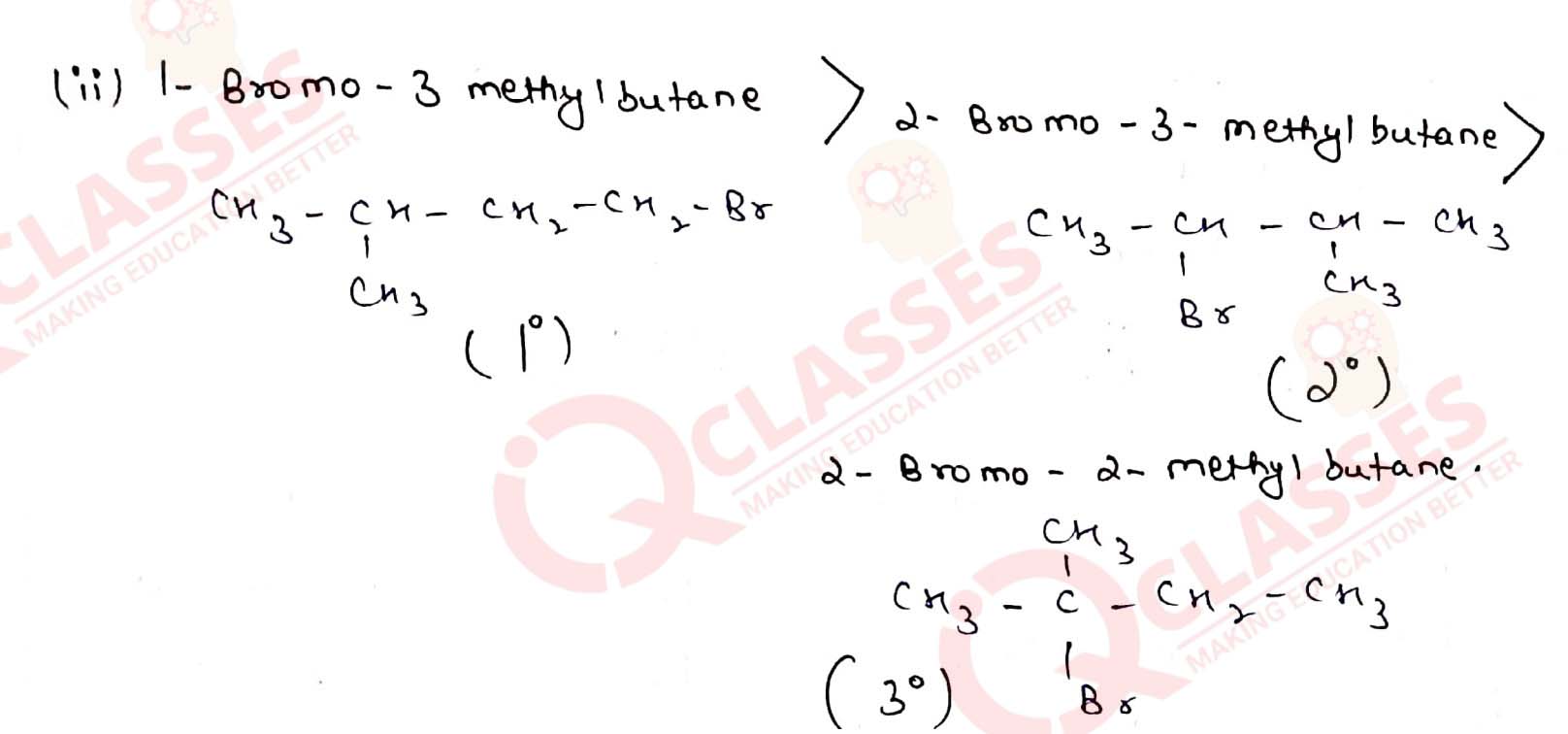
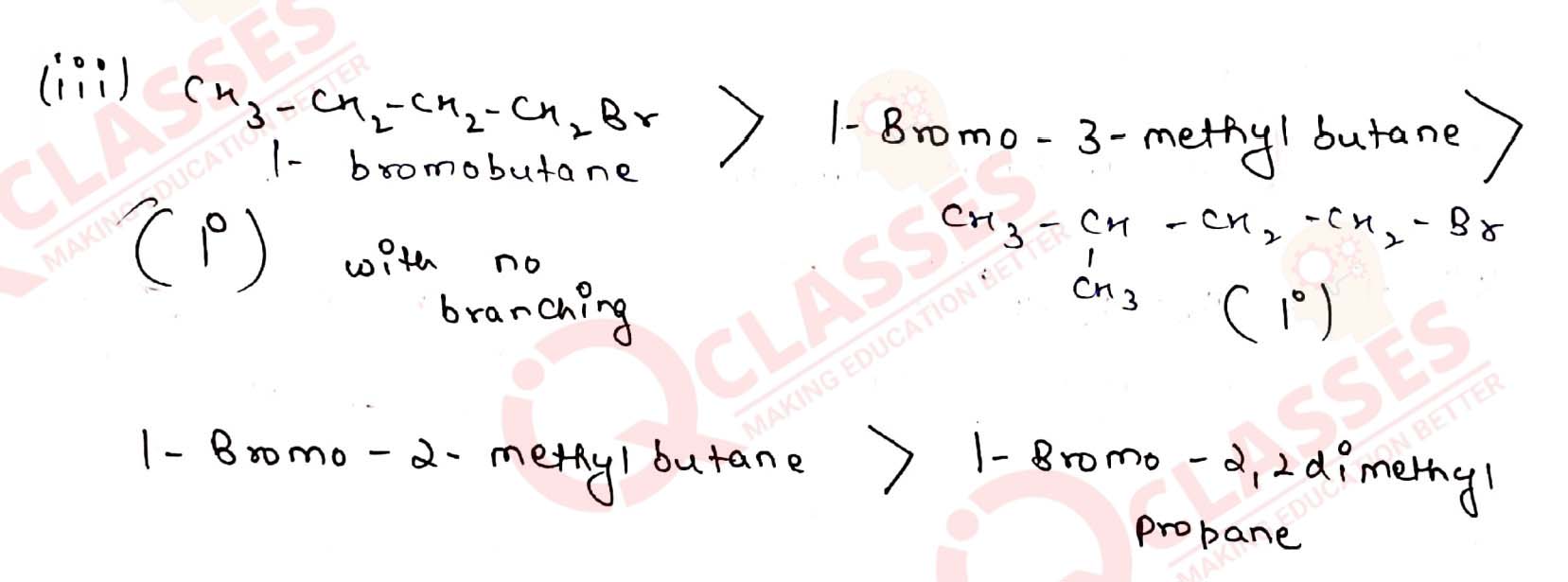

(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
(iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane.
Solution