Q2.1
Which of the following units do not vary with change in temperature:
molarity, molality, normality, mole fraction?
Solution
molarity and mole fraction these units do not vary with change in temperature.
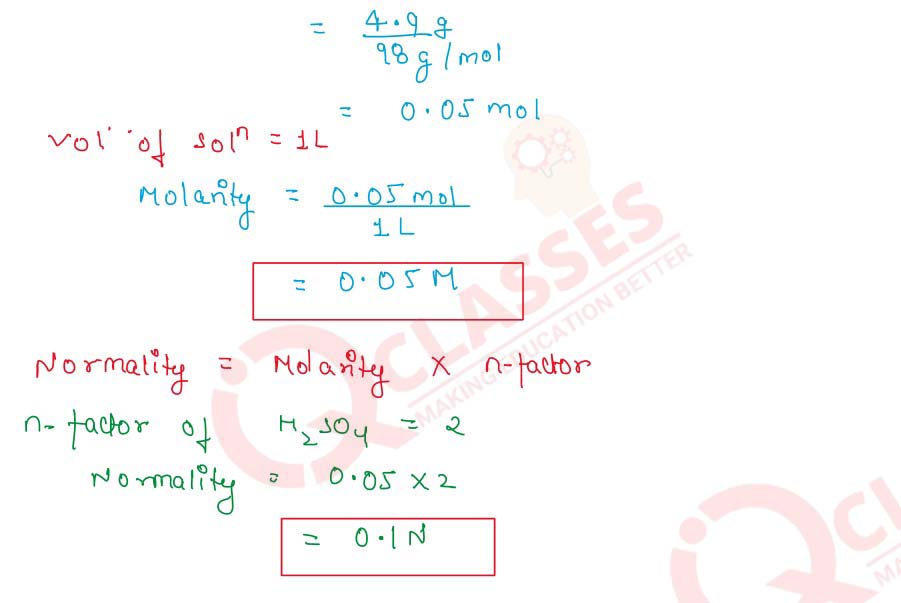
Q2.2 Calculate the normality and molarity of H2SO4 solution containing 4.9 g of H2SO4 per litre of the solution
Solution
Molarity=
Number of Moles of Solute
/
Vsolution(L)
Molar Mass of H2SO4 =98 g/mol
Given Mass of H2SO4=4.9 g
Number of moles=
Given Mass
/
Molar Mass
Q2.3 6 g of NaOH are dissolved in 200 cm3 of water. What is the relation between molarity and normality of the solution thus obtained?
Solution
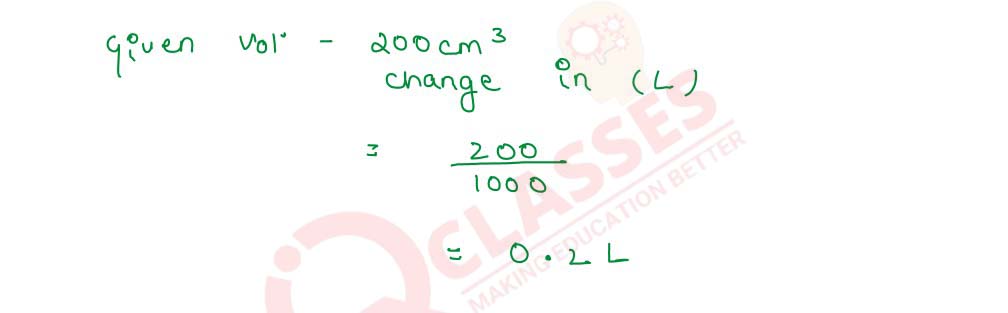
Molarity=
Number of Moles of Solute
/
Vsolution(L)
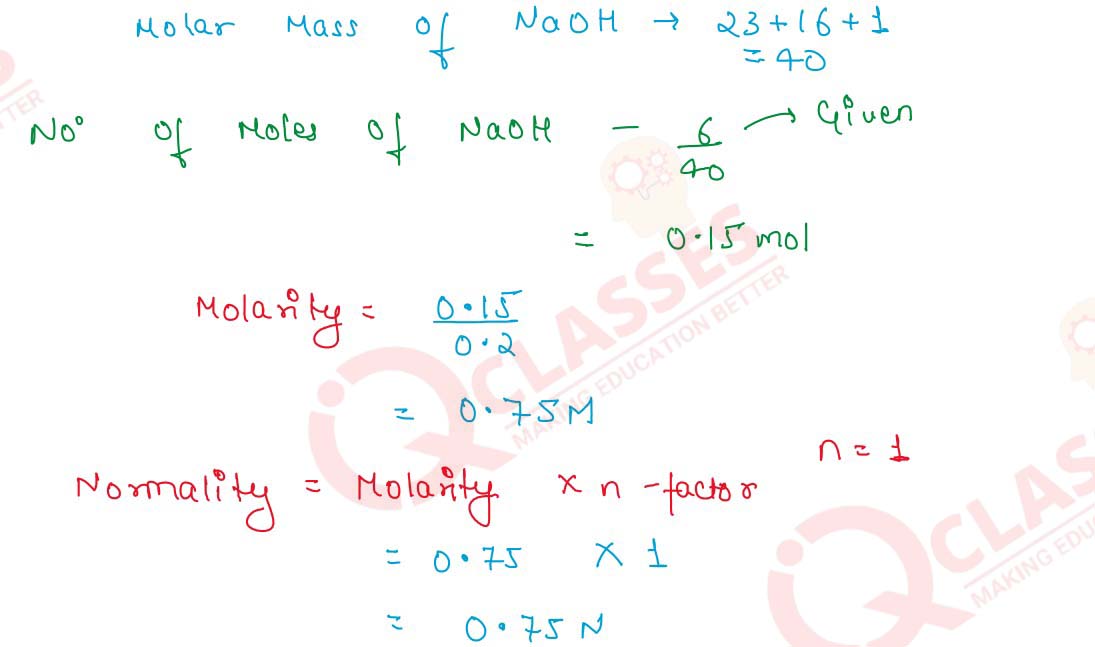
Hence,
Molarity and Normality are same
Q2.4
Calculate the mass of the solute in the following solutions :
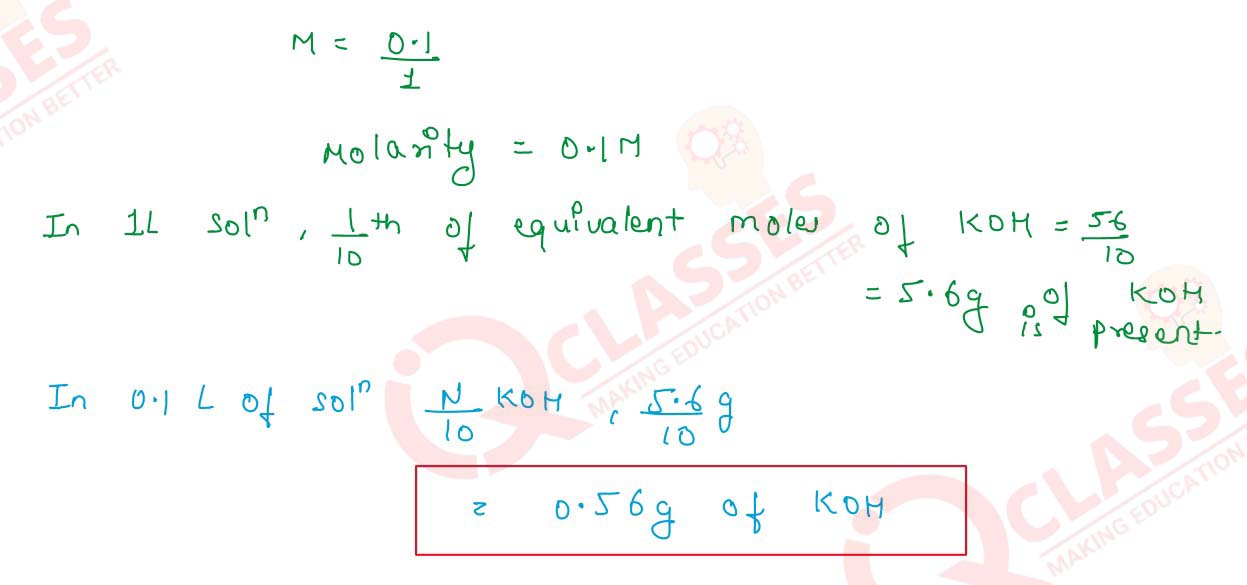
- 100 cm3 of N/10 KOH
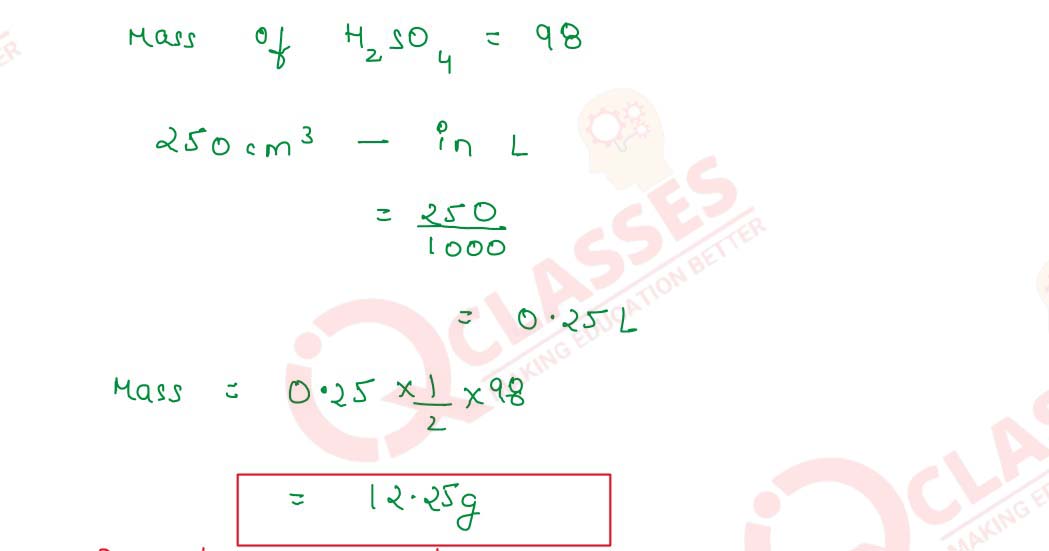
- 250 cm3 of N H2SO4
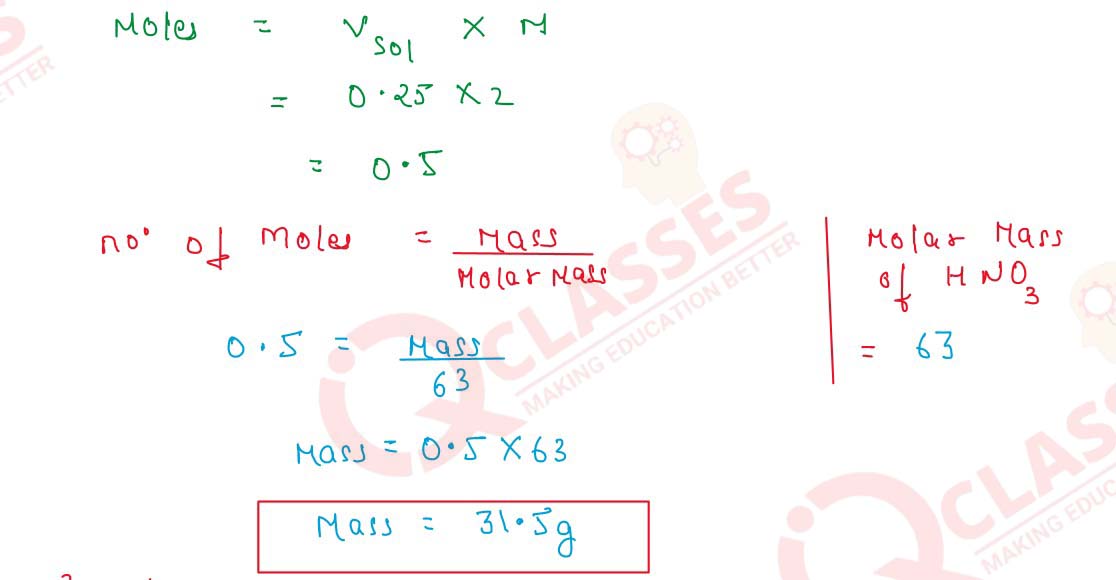
- 250 cm3 of 2M HNO3
- 150 cm3 of M/2 HCl
Solution

Molarity=
Normality
/
n-factors
(ii) 250 cm3 of N H2SO4
(iii) 250 cm3 of 2M HNO3
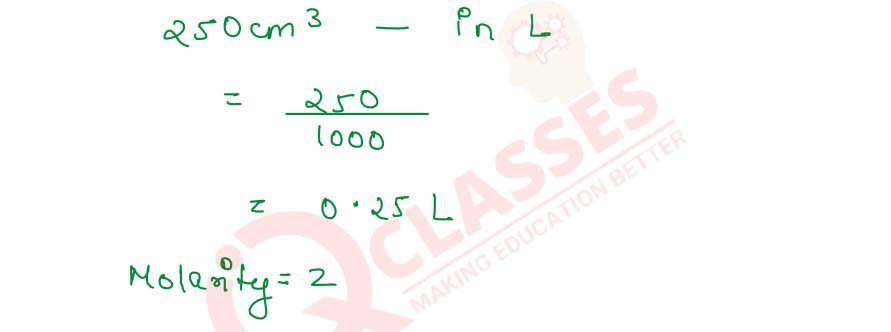
Molarity=
Number of Moles of Solute
/
Vsolution(L)
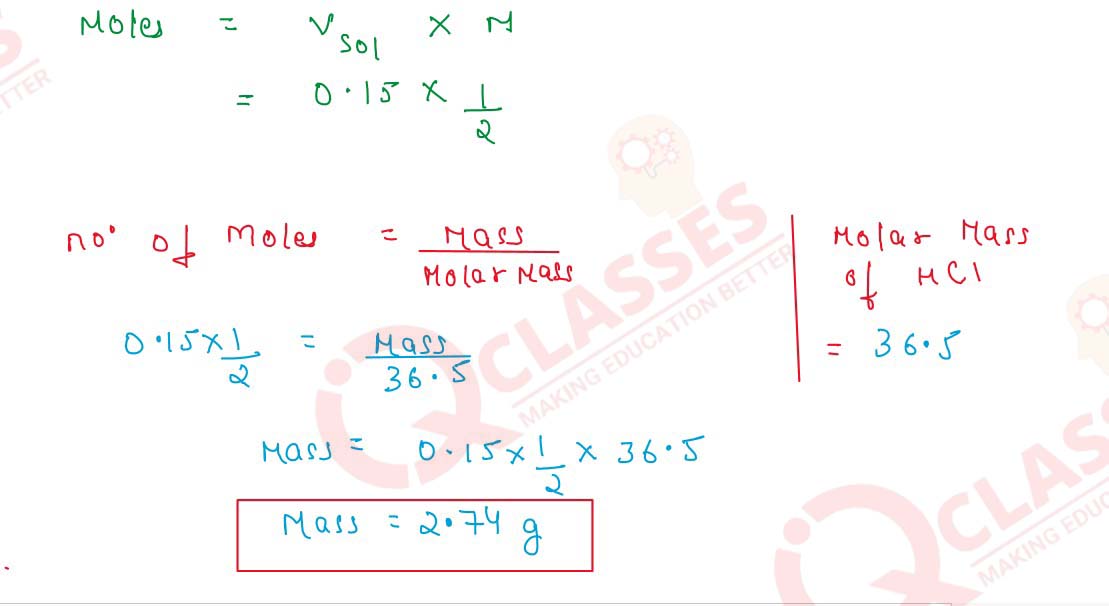
(iv) 150cm3 of m/2 HCl

Molarity=
Number of Moles of Solute
/
Vsolution(L)
Q2.5 5.85 g of NaCl are dissolved in 500 cm3 of water. Calculate the formality of the solution.
Solution
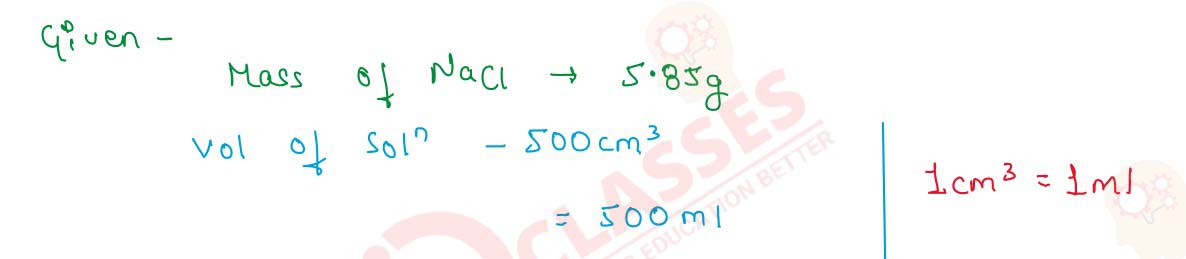
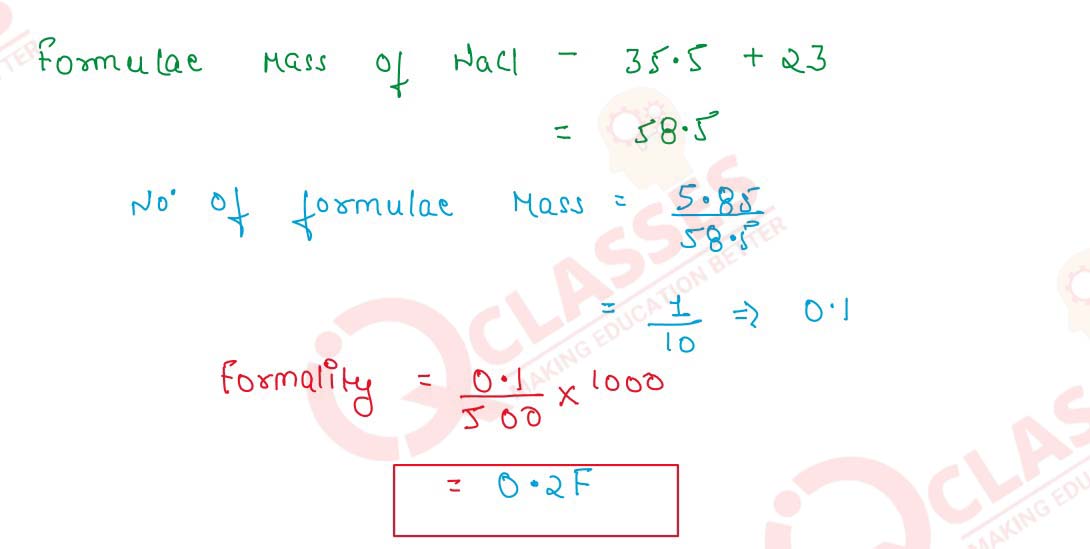
Formality=
Number of formulae Mass
/
Volume of Solution(mL)
X100
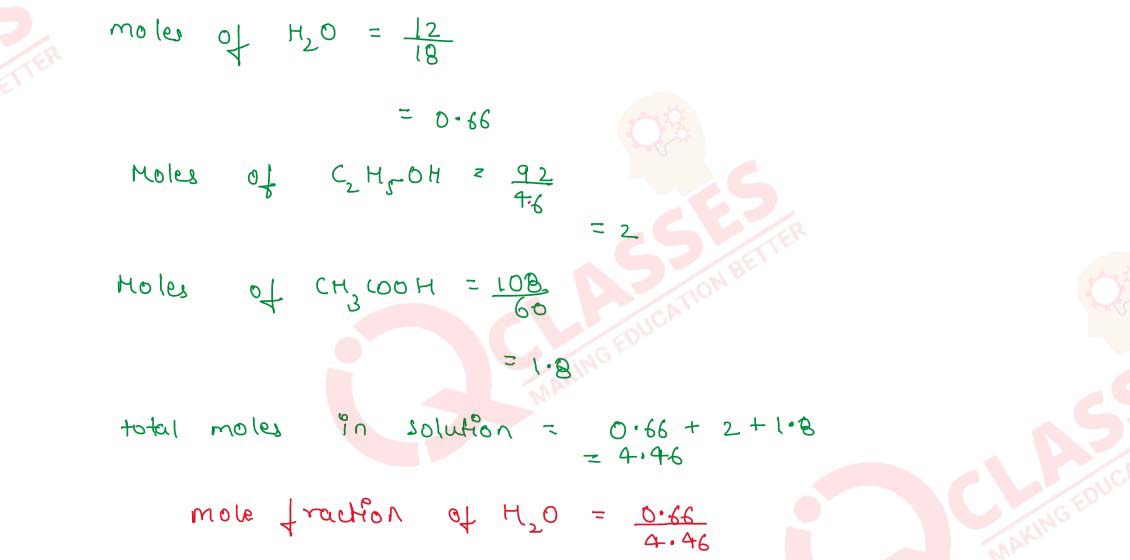
Q2.6 Calculate the mole fraction of water in a mixture of 12g of water, 108g of acetic acid and 92g of ethyl alcohol.
Solution
Number of moles of compound=
mass in grams
/
molecular weight

Q2.7 2.46 g of sodium hydroxide (molar mass = 40) are dissolved in water and the solution is made to 100 cm3 in a volumetric flask. Calculate the molarity of the solution
Solution
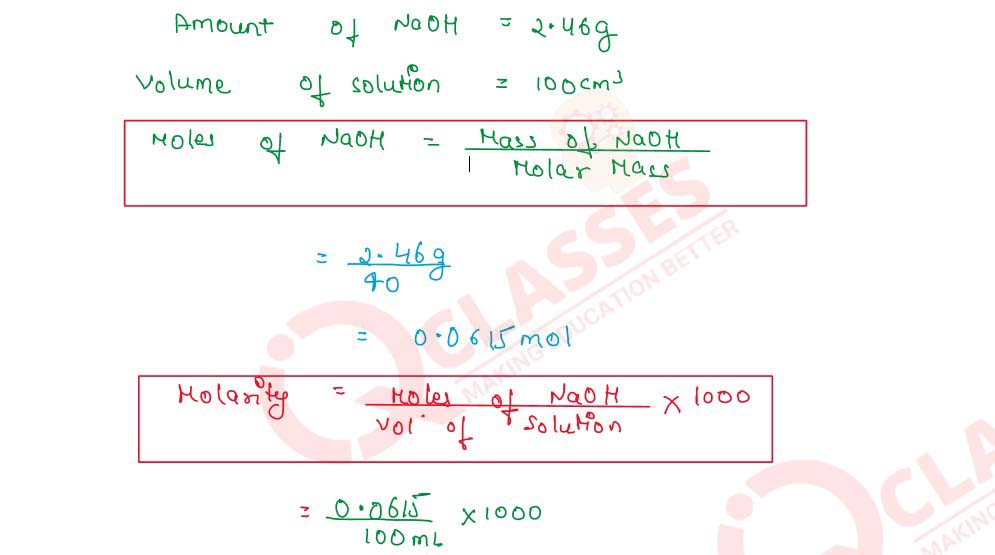

Q2.8 Concentrated nitric acid used as a laboratory reagent is usually 69% by mass of nitric acid. Calculate the volume of the solution which contained 23 g of HNO3. Density of conc. HNO3 solution is 1.41 g cm -3
Solution
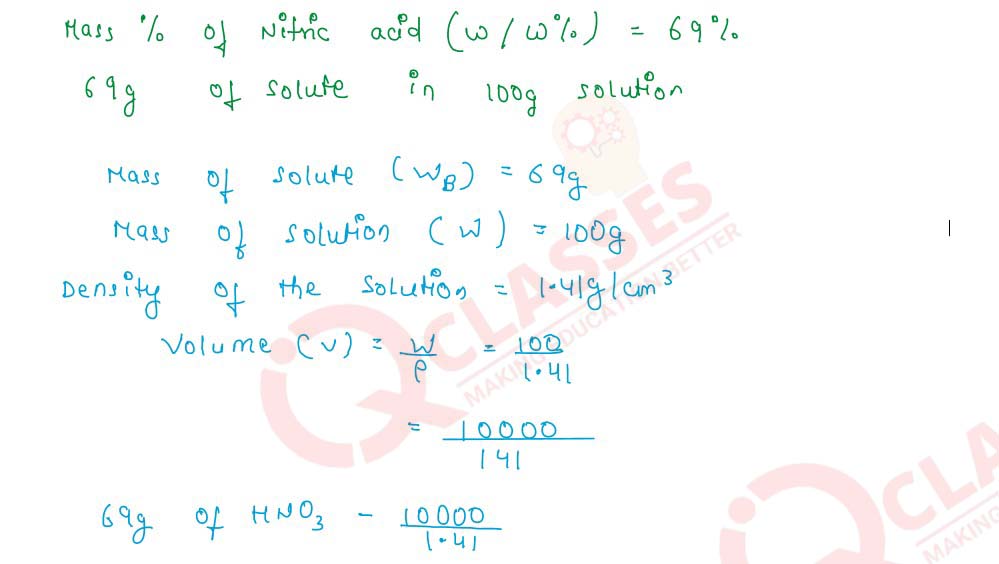
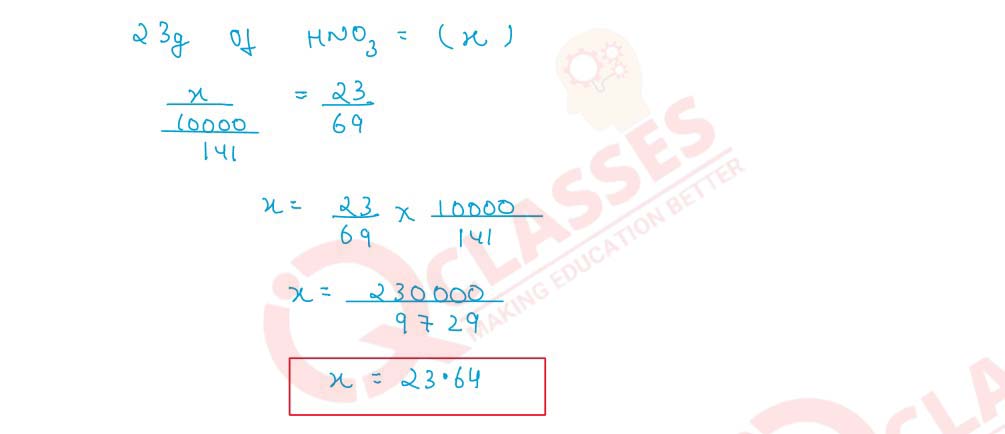
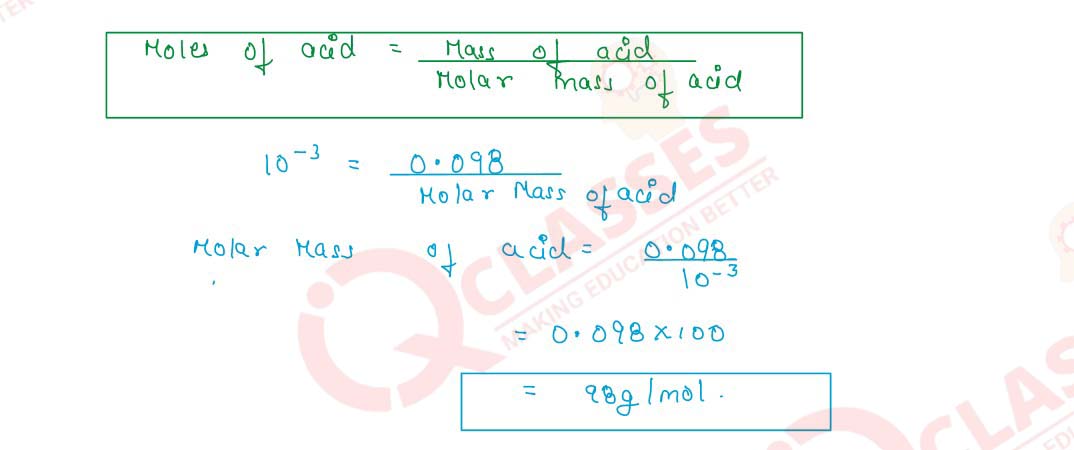
Q2.9 100 cm3 of a centimolar solution of an acid contain 0.098g of the acid. Find the molecular mass of the acid.
Solution
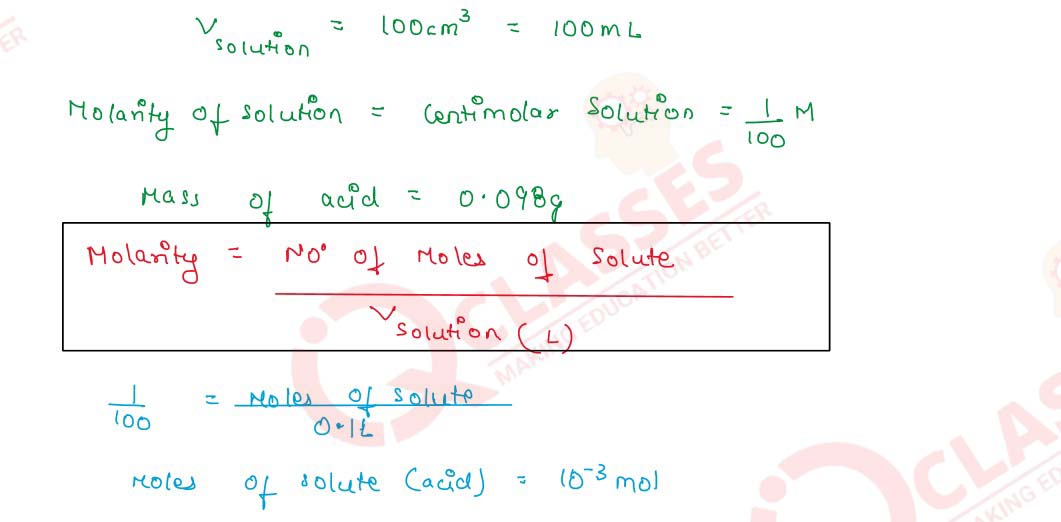
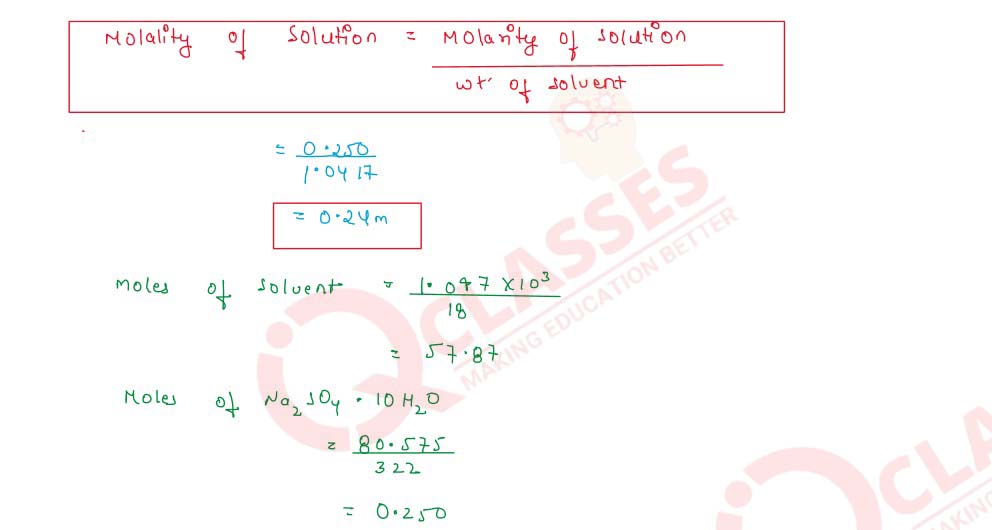
Q2.10 8.0575 x 10-2 kg of Glauber's salt are dissolved in water to obtain 1 cm3 of a solution of density 1077.2 kg m-3. Calculate the molarity, molality, and mole fraction of Na2SO4 in the solution
Solution



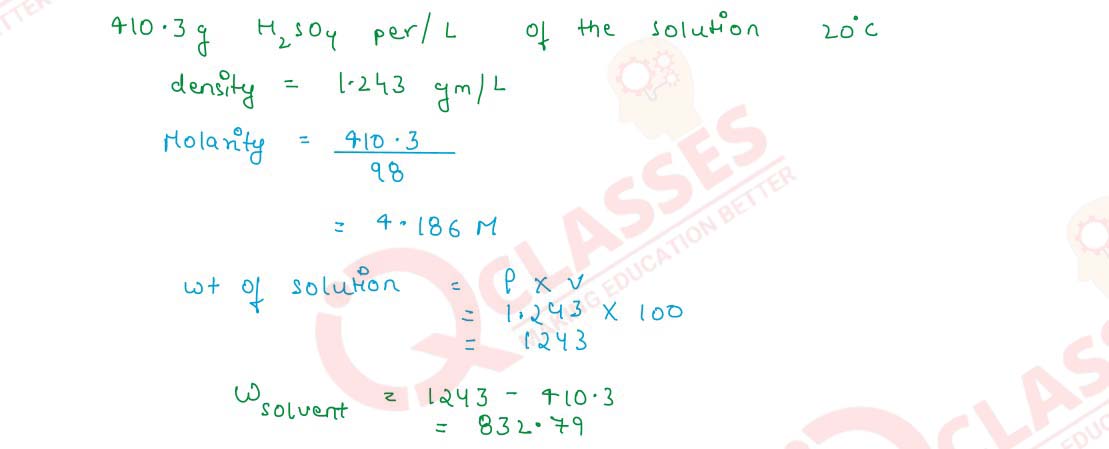
Q2.11 A solution contains 410.3 g of H2SO4 per litre of the solution at 20°C. If its density is 1.243 g cm-3, what will be its molality and molarity?
Solution

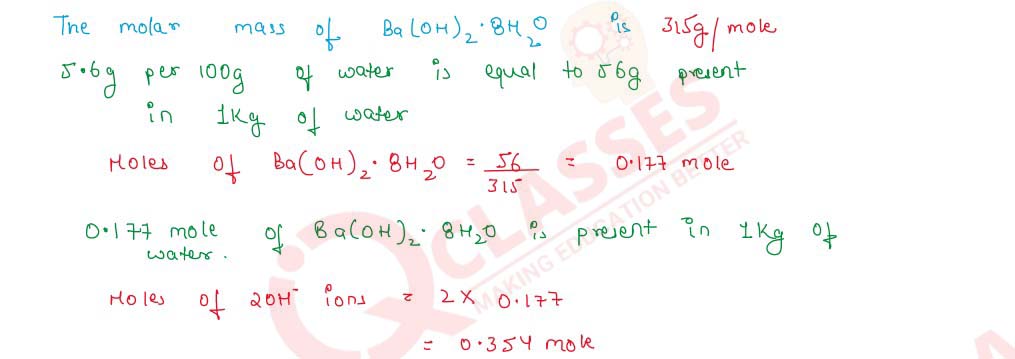
Q2.12 The solubility of Ba(OH)2.8H2O in water at 288 K is 5.6 g per 100 g of water. What is the molality of the hydroxide ions in the saturated solution of barium hydroxide at 288 K? (Atomic masses : Ba = 137, 0 = 16, H = 1)
Solution
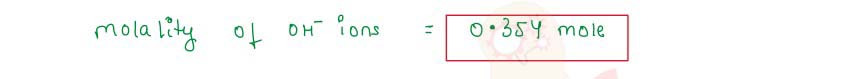
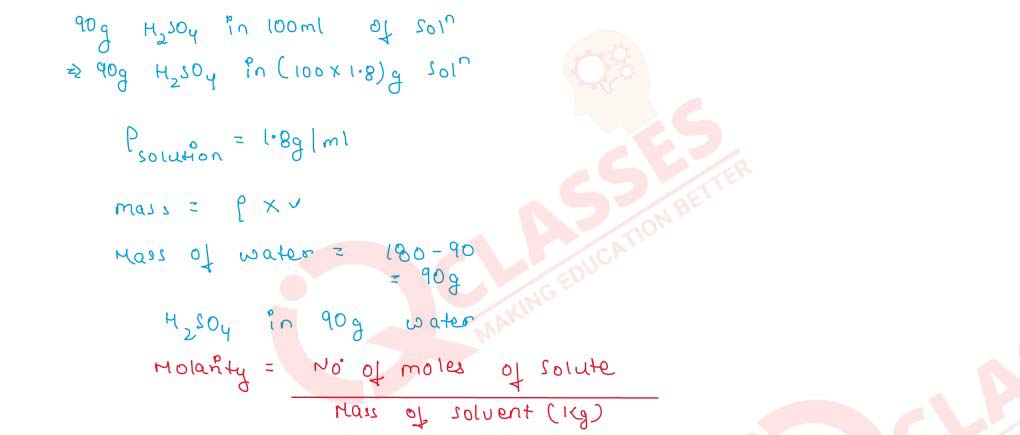
Q2.13 Calculate the molality of 1 litre solution of 90% H2SO4 (weight/volume). The density of solution is 1.80 g mL-1
Solution

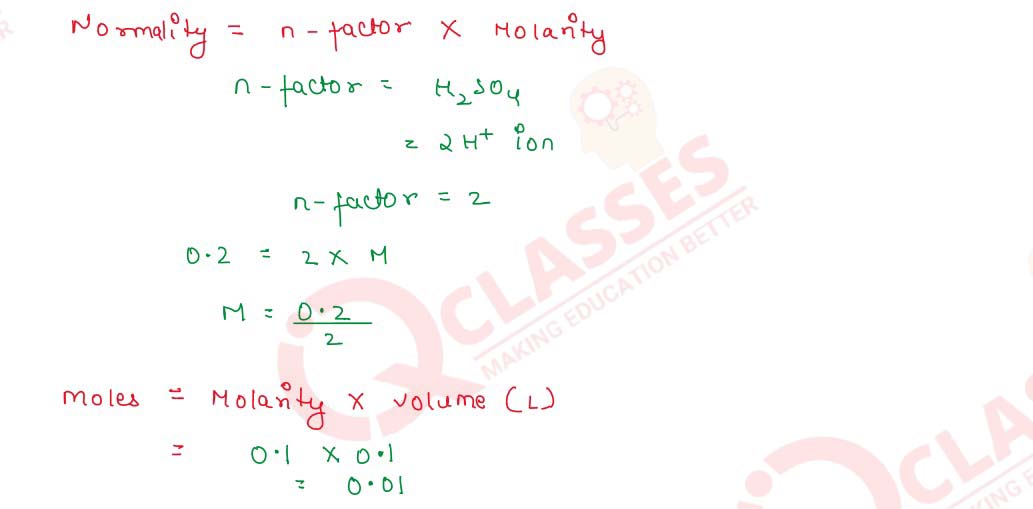
Q2.14 Calculate the number of molecules of oxalic acid (H2C2O4.2H20) in 100 mL of 0.2N oxalic acid solution.
Solution
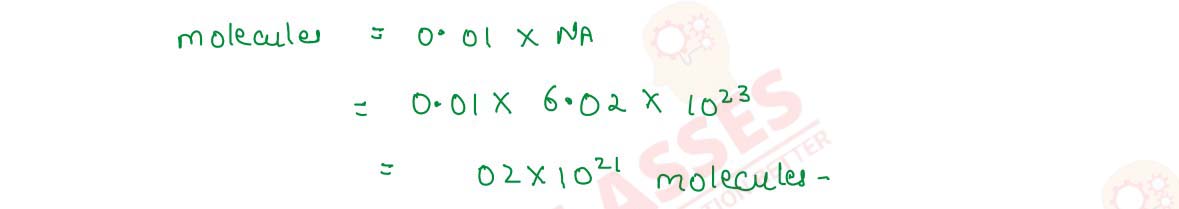
Q2.15 150 cm3 of a decimolar NaOH solution are diluted to 750 cm3. Find the molarity of the diluted solution
Solution
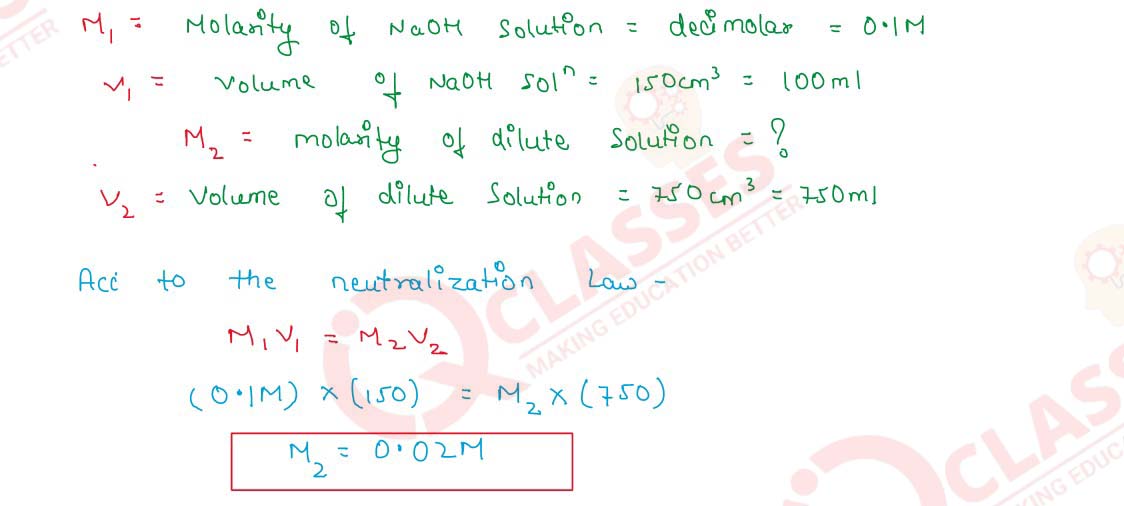
Q2.16 One tonne of air contains 2 x 10-3 g of carbon as smoke. Calculate the concentration of carbon in ppm in air.
Solution


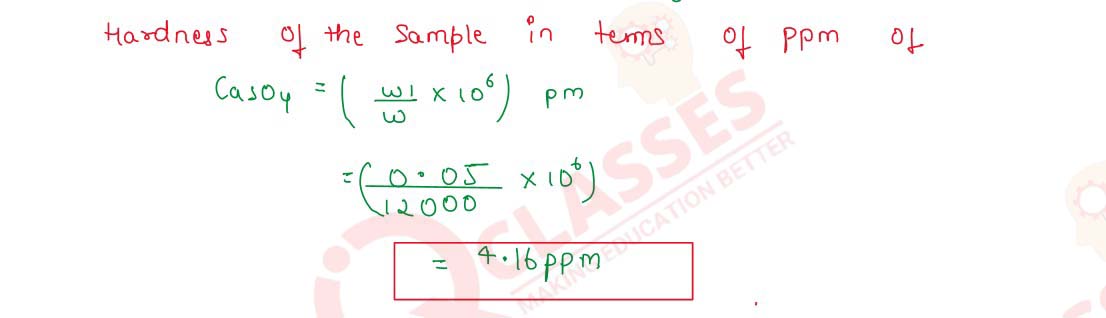
Q2.17 A sample Of hard water is found to contain 50 mg of CaSO4 in 12 kg of the sample. Calculate the hardness of the sample in terms of ppm of CaSO4.
Solution
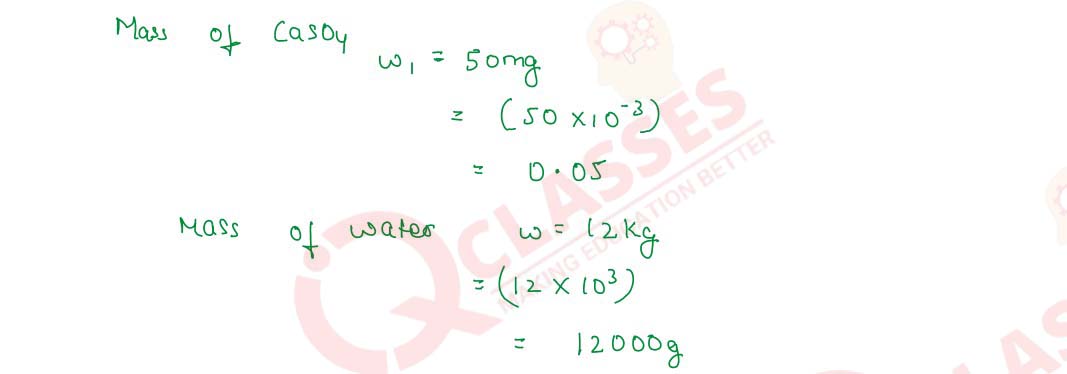
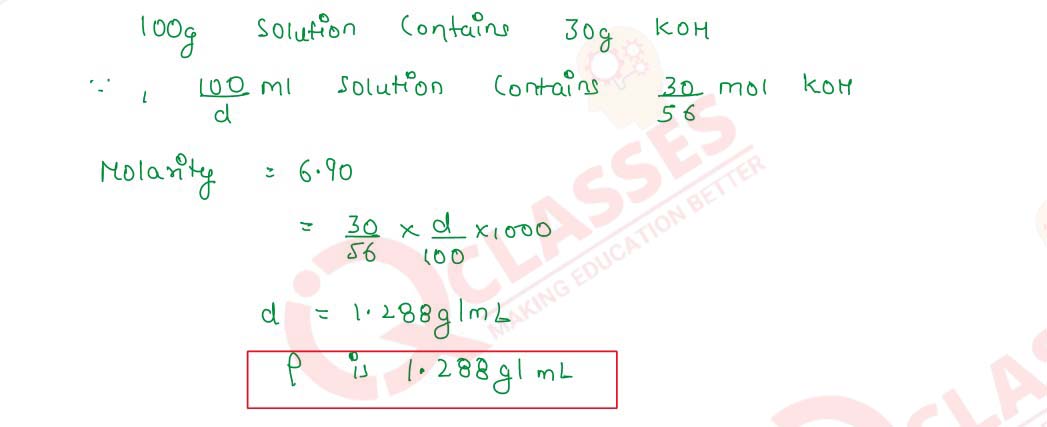
Q2.18 A 6.90 M solution of KOH in water contains 30% by weight of KOH. What is the density of the solution?
Solution
Q2.19 Define the terms molarity and molality for a solution. How does a rise in temperature change the molality and molarity values of the solution?
Solution
Molarity
Molarity is defined as the number of Moles
of solute present in 1L of solution.
Molarity=
Number of Moles of Solute
/
Vsolution(L)
Molality
Molality is defined as the number of moles of solute present in 1kg of solvent.
Molality=
Moles of Solute
/
Mass of solvent(kg)
Molarity is temperature dependent, volume increase with increase in temperature
but, molality this unit consist of mass and is independent on the temperature.
Q2.20 Calculate the molarity of a solution of 93% H2SO4 (w/V) when the density of the solution is 1.84 g cm-3.
Solution

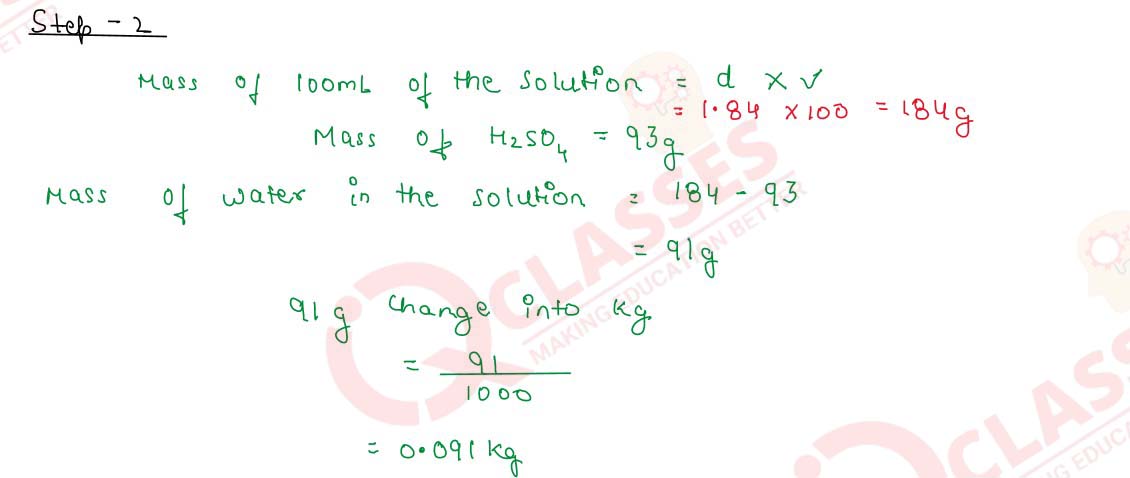

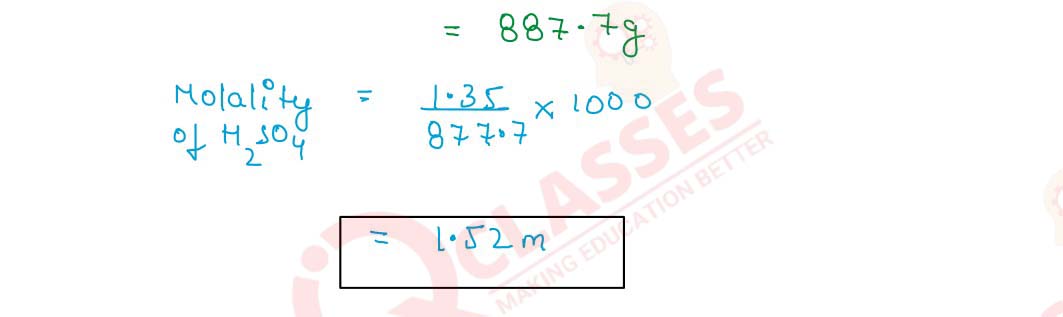
Q2.21 The molarity of a solution of sulphuric acid is 1.35 M. Calculate its molality. (The density of the acid solution is 1.02 gcm-3
Solution
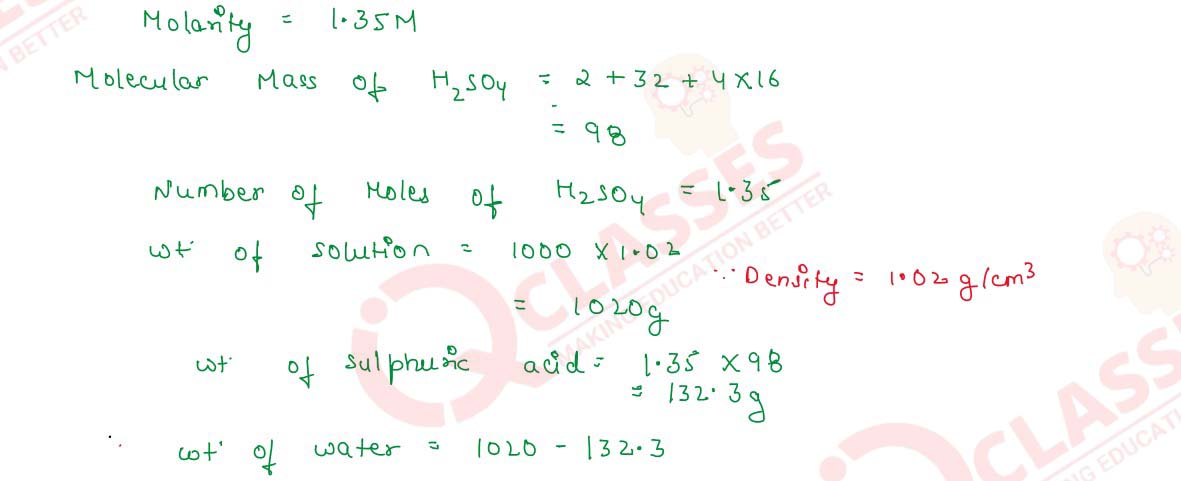
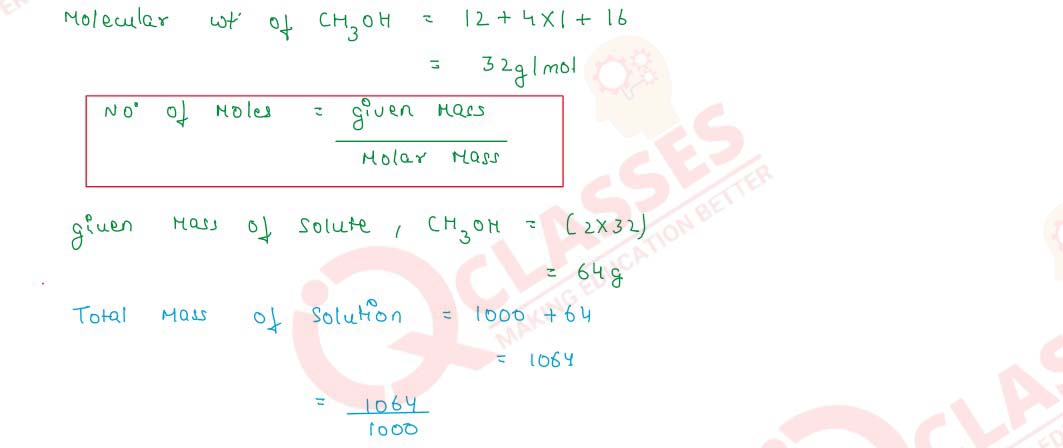
Q2.22 Calculate the number of moles of methanol in 5 litres of its 2 m solution, if the density of the solution is 0.981 kg L-1 (Molar mass of methanol = 32.0 g mol-1).
Solution
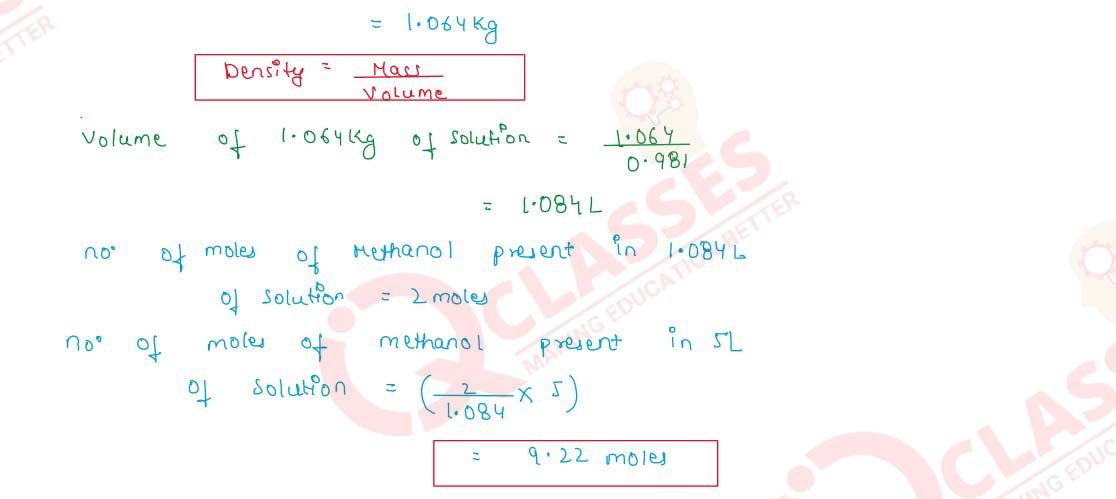
Q2.23 How is molality of a solution different from its molarity
Solution
Molarity
Molarity is defined as the number of Moles
of solute present in 1L of solution.
Molarity=
Number of Moles of Solute
/
Vsolution(L)
Molality
Molality is defined as the number of moles of solute present in 1kg of solvent.
Molality=
Moles of Solute
/
Mass of solvent(kg)
Molarity is temperature dependent, volume increase with increase in temperature
but, molality this unit consist of mass and is independent on the temperature.
Q2.24 What is the sum of the mole fractions of all the components in a three component system.
Solution
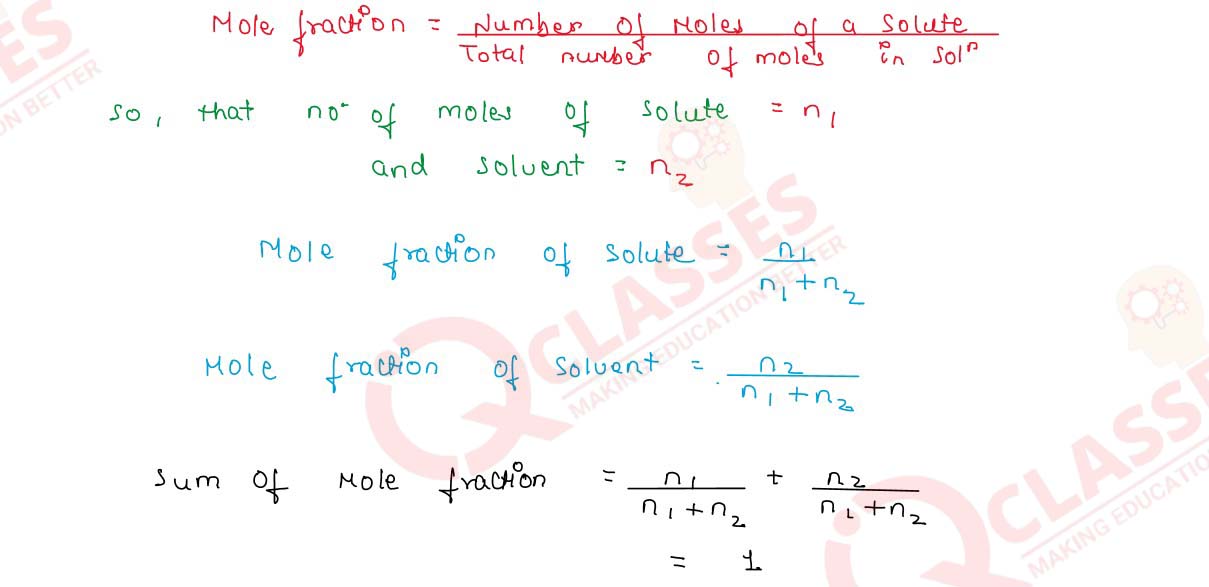
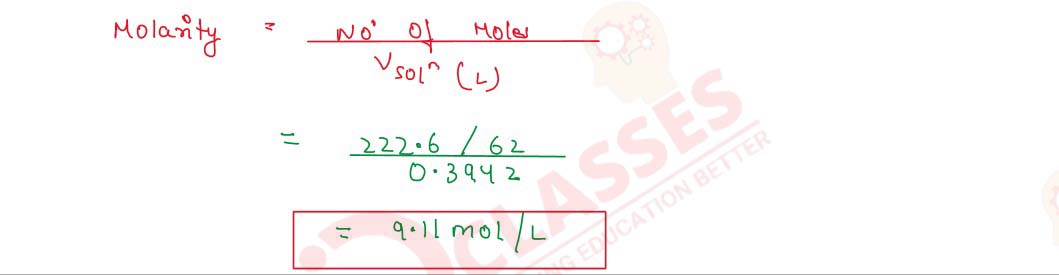
Q2.25 An antifreeze solution is prepared from 222.6 g of ethylene glycol C2H4(OH)2 and 200 g of water. Calculate the molality of the solution. If the density of this solution be 1.072 g mL-1, what will be the molarity of the solution?
Solution