1
(a) What: is a chemical reaction?
(b) State the conditions necessary for a chemical change or reaction.
Solution
(b) State the conditions necessary for a chemical change or reaction.
Solution

2
Define the following terms
(a) Chemical change
(b) Chemical bond
(c) Effervescence
(d) Precipitate
Solution
(a) Chemical change
(b) Chemical bond
(c) Effervescence
(d) Precipitate
Solution

3
Give an example of a reaction where the following are involved
(a) Heat (b) Light (c) Electricity (d) Close contact (e) Solution (f) Pressure (g) Catalyst
Solution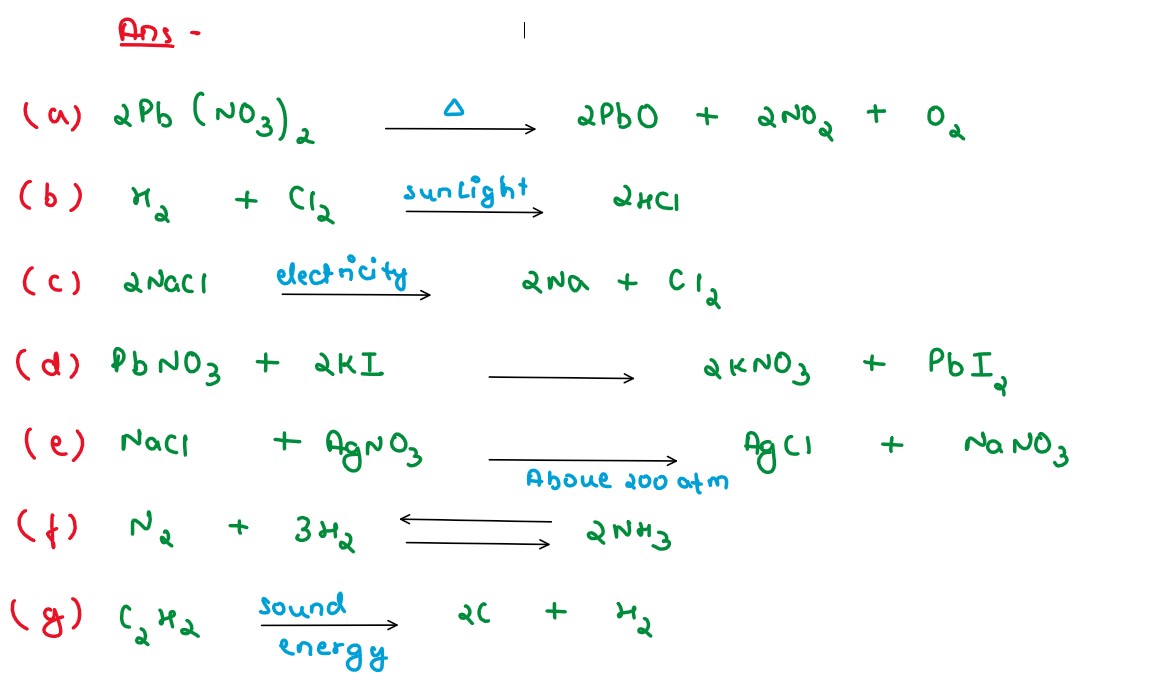
(a) Heat (b) Light (c) Electricity (d) Close contact (e) Solution (f) Pressure (g) Catalyst
Solution

4
Define :
(a) Photochemical reaction (b) Electrochemical reaction. Give one example in each
Solution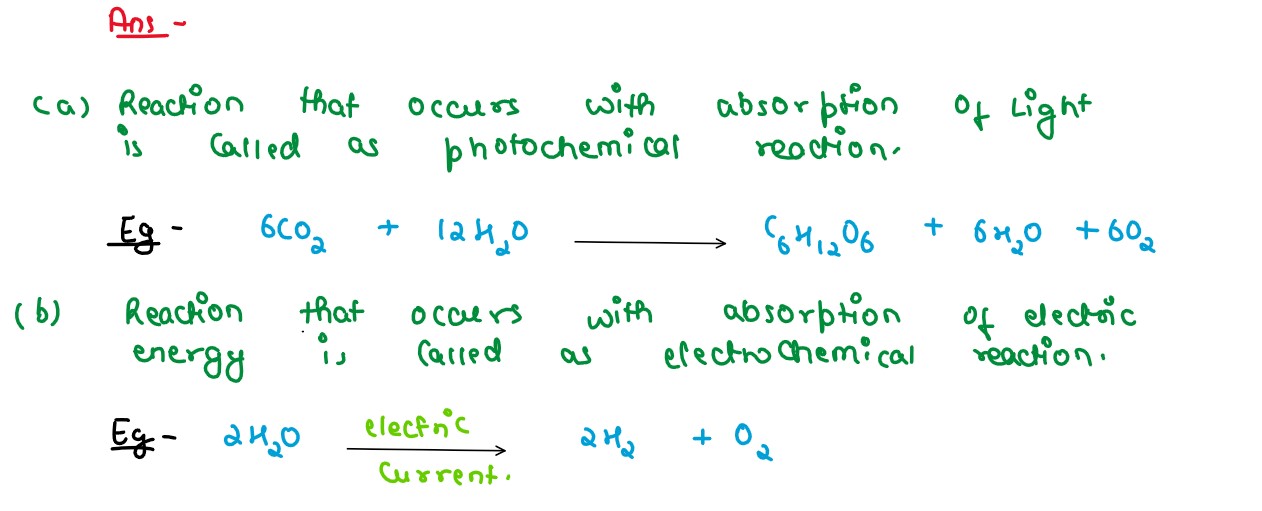
(a) Photochemical reaction (b) Electrochemical reaction. Give one example in each
Solution

5
Give an example of each of the following chemical changes.
(a) A photochemical reaction involving
(i) silver salt (ii) water
(b) A reaction involving
(i) blue solution
(ii) formation of a dirty green precipitate
(c) Two gases combine to form a white solid.
(d) Two solids combine to form a liquid.
(e) A reaction where color change is noticed.
Solution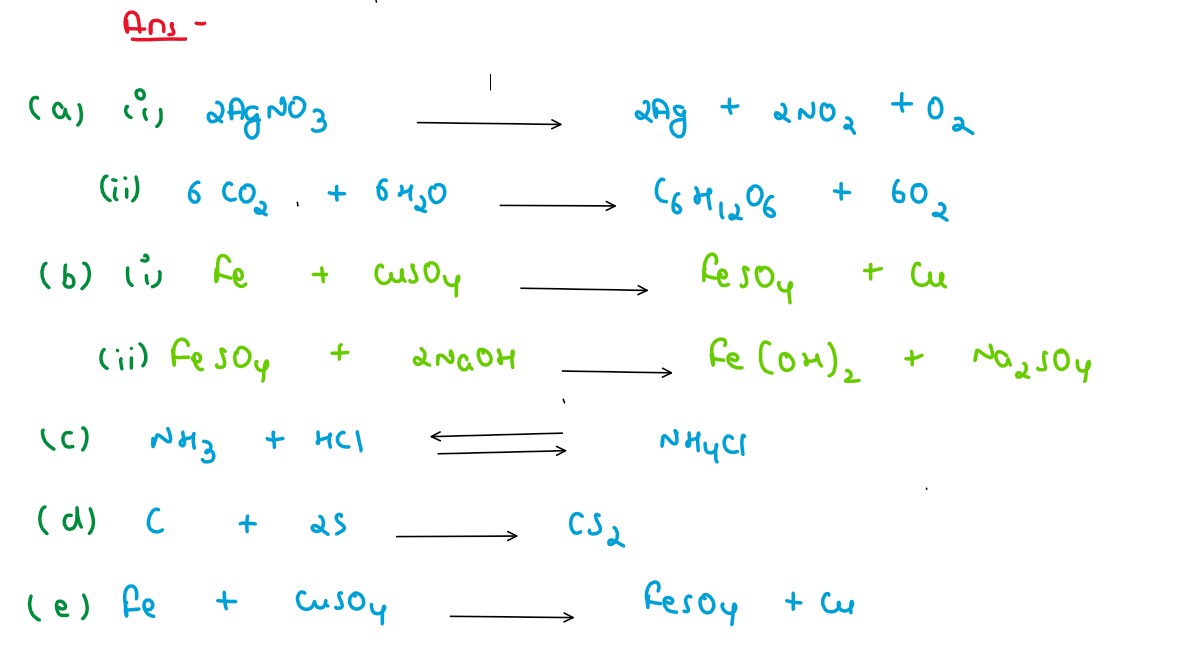
(a) A photochemical reaction involving
(i) silver salt (ii) water
(b) A reaction involving
(i) blue solution
(ii) formation of a dirty green precipitate
(c) Two gases combine to form a white solid.
(d) Two solids combine to form a liquid.
(e) A reaction where color change is noticed.
Solution
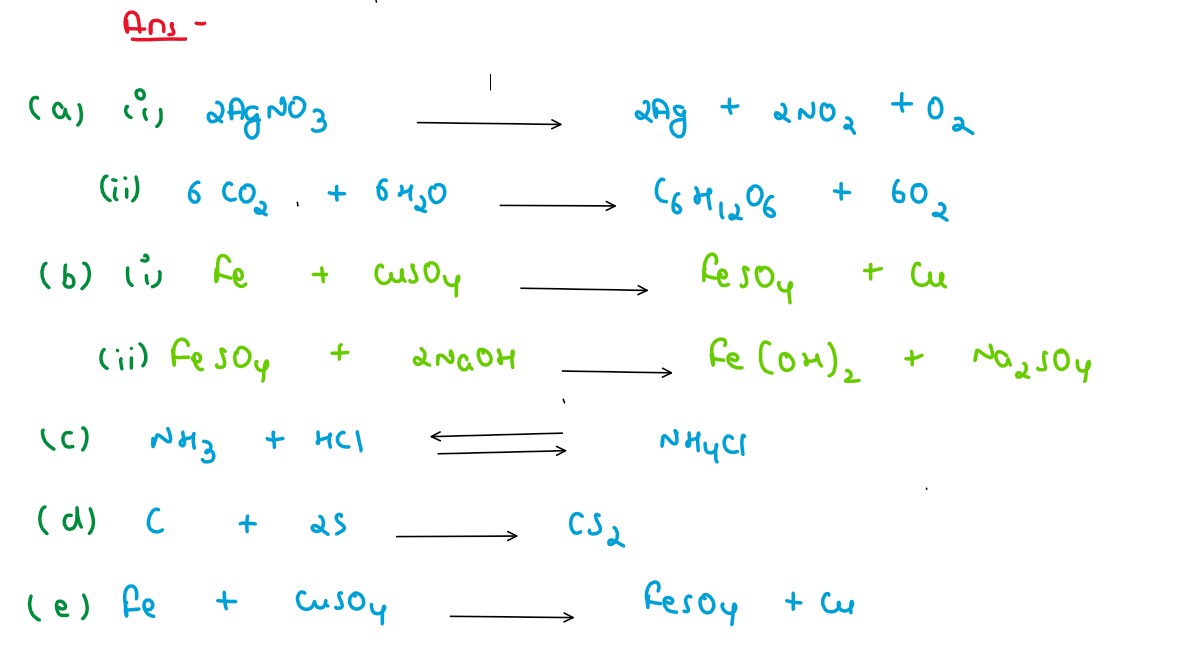
6
Write the chemical reaction where the following changes observed.
a) Gas is evolved
b) Colour change is noticed
c) Precipitate is formed
d) Physical state is changed
Solution
a) Gas is evolved
b) Colour change is noticed
c) Precipitate is formed
d) Physical state is changed
Solution

7
Give a reason for the following :
(a) Silver nitrate solution is kept in coloured bottles.
(b) Molybdenum is used in the manufacture of ammonia.
(c) A blue solution of copper sulphate changes to green when a piece of iron is added to this solution.
(d) Colourless concentrated sulphuric acid in a test tube changes to blue on adding a small piece of copper to it.
Solution
(a) Silver nitrate solution is kept in coloured bottles.
(b) Molybdenum is used in the manufacture of ammonia.
(c) A blue solution of copper sulphate changes to green when a piece of iron is added to this solution.
(d) Colourless concentrated sulphuric acid in a test tube changes to blue on adding a small piece of copper to it.
Solution
