Q1
What is the need for classification of elements?
Solution

Q2
What was the basis of the earliest attempts made for classification and grouping of elements?
Solution


Q3
(a) A, B and C are the elements of a Dobereiner’s triad. if the atomic mass of A is 7 and that of C is
39, what should be the atomic mass of B?
(b) Why was Dobereiner’s triad discarded?
Solution
(b) Why was Dobereiner’s triad discarded?
Solution

Q4
Explain ‘Newland’s law of Octaves.’ Why was the law discarded?
Solution


Q5
Did Dobereiners triads also exist in the columns of Newland’s Octaves? Compare and find out.
Solution


Q6
(a) Lithium. sodium and potassium elements were put in one group on the basis of their similar
properties. What are those similar properties
(b) The elements calcium, strontium and barium were put in one group or family on the basis of their similar properties. Whit were those similar properties? Solution

(b) The elements calcium, strontium and barium were put in one group or family on the basis of their similar properties. Whit were those similar properties? Solution


Q7
(a) What was Mendeleev’s basis for classification of elements?
(b) Mendeleev’s contributions to the concept of a periodic table laid the foundation for the Modern Periodic Table. Give reasons. Solution
(b) Mendeleev’s contributions to the concept of a periodic table laid the foundation for the Modern Periodic Table. Give reasons. Solution

Q8
State Mendeleev’s periodic law.
Solution


Q9
Use Mendeleev’s periodic Law to predict the formula of
(a) hydrides of carbon and silicon
(b) Oxides of potassium, aluminium and barium Solution
(a) hydrides of carbon and silicon
(b) Oxides of potassium, aluminium and barium Solution

Q10
Which group of elements was missing from Mendeleev’s original periodic table ?
Solution


Q11
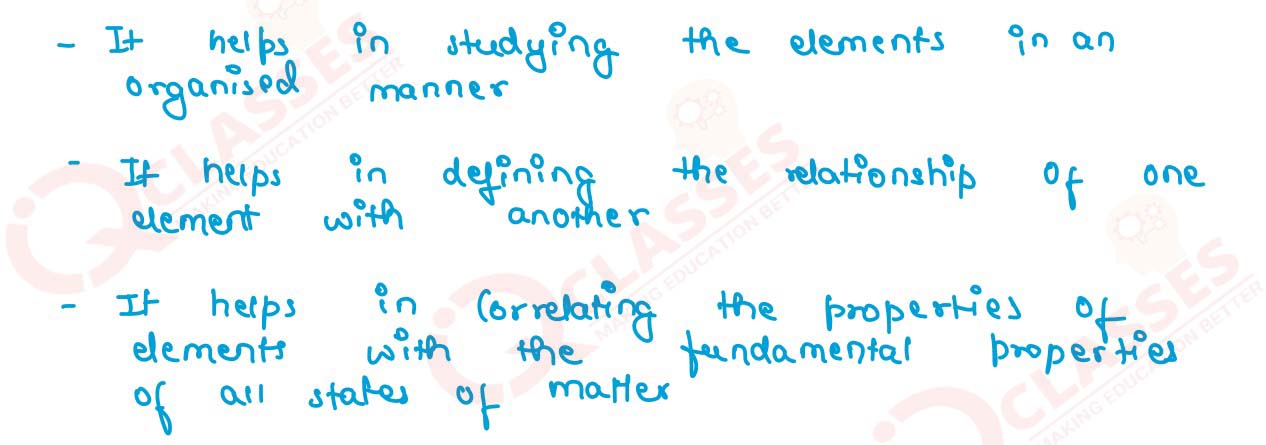
State the merits of Mendeleev’s classification of elements.
Solution
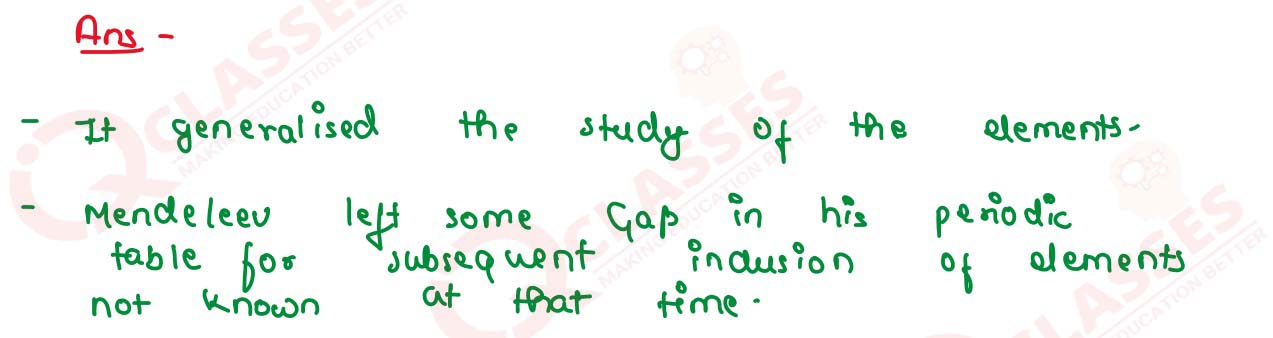
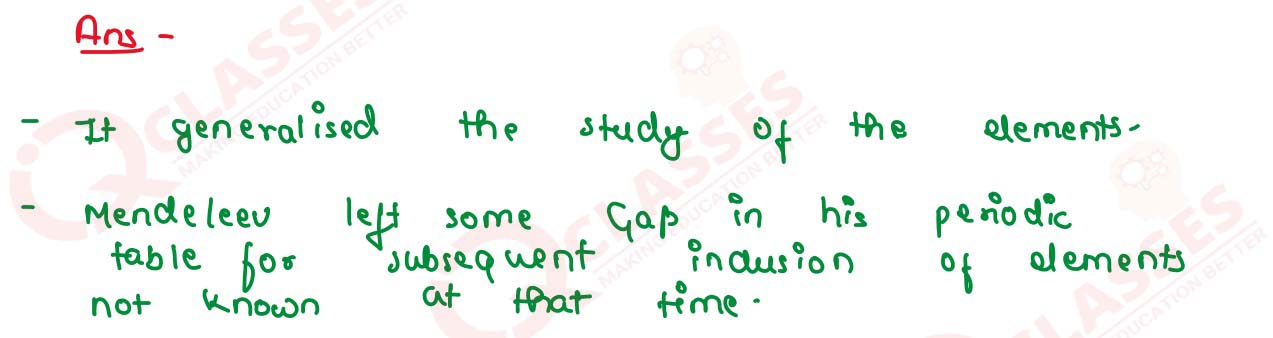
Q12
Why did Mendeleev leave some gaps in his periodic table of elements? Explain your answer with an
example.
Solution
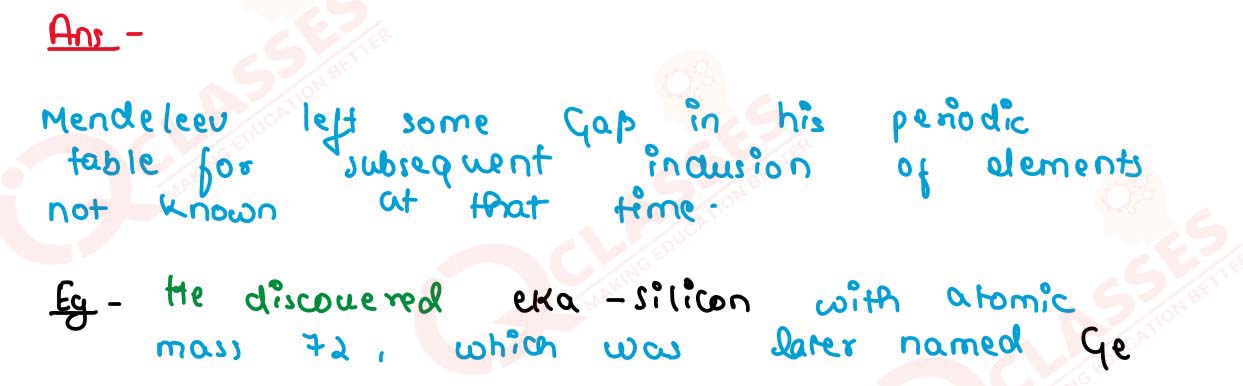
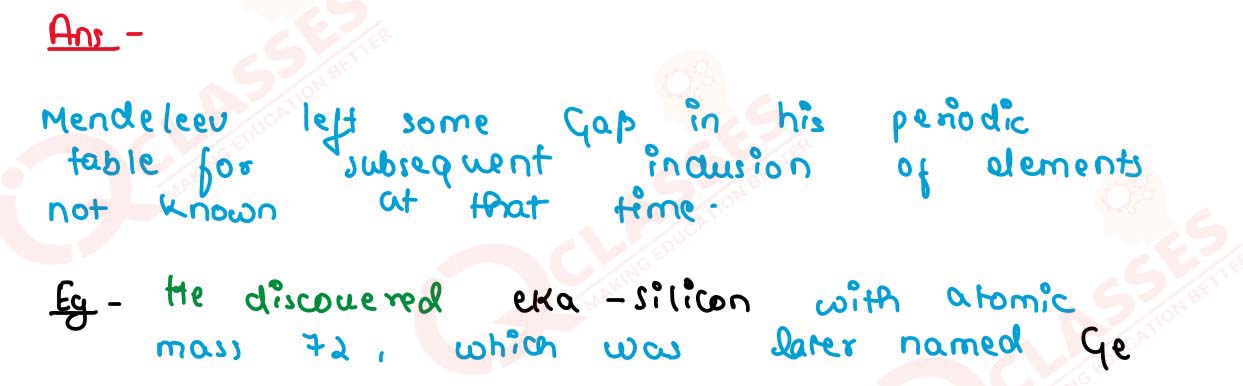
Q13
The atomic number of an element is more important to the chemist than its relative atomic mass. Why?
Solution


Q14
Consider the following elements: Be, Li, Na, Ca, K. Name the elements of (a) same group (b) same period.
Solution


Q15
(a) Name an element whose properties were predicted on the basis of its position in Mendeleev’s periodic
table.
(b) Name two elements whose atomic weights were corrected on the basis of their positions in Mendeleev’s periodic table.
(c) How many elements were known at the time of Mendeleev’s classification of elements? Solution
(b) Name two elements whose atomic weights were corrected on the basis of their positions in Mendeleev’s periodic table.
(c) How many elements were known at the time of Mendeleev’s classification of elements? Solution
