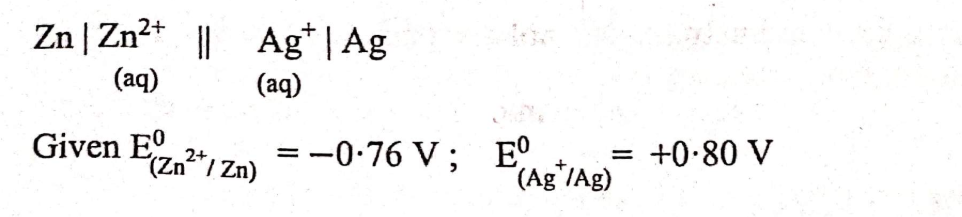This paper is divided into four sections — A, B, C and D.
Answer all questions.
Section — A consists of one question having sub-parts of one mark each.
Section — B consists of ten questions of two marks each.
Section — C consists of seven questions of three marks each, and
Section — D consists of three questions of five marks each.
Internal choices have been provided in one question each in Section B,
Section C and Section D.
All working, including rough work, should be done on the same sheet as, and adjacent to the
rest of the answer.
Question
1
(A) Fill in the blanks by choosing the appropriate word(s) from those given below in
the brackets.
[lead poisoning, zero, phosgene, dependent, cancer, independent, diethyl ether,
first, ethyl carbonate, ethene]
(i) For a particular reaction, the value of rate constant is 0.05 sec-1. The reaction is of _____ order and will be ____ of the initial concentration.
View Solution
(ii) EDTA is used in the treatment of _____ while Cisplatin is used in the treatment of _____
View Solution
(iii) The addition of small quantity of ethanol to chloroform prevents the formation of ______ and converts it into the harmless compound ______
View Solution
(iv) The Dehydration of ethyl alcohol with conc. H2SO4 at 140°C mainly yields ______ while at 170°C the main product formed is ______
View Solution
(B) Select and write the correct alternative from the choices given below
(i) Which one of the following statements is correct regarding the dry cell?
(P) Zinc container acts as an anode in dry cell.
(Q) Zinc container touches the paste of MnO2 and carbon.
(R) Dry cell can be charged easily.
(S) Graphite rod acts as a cathode in dry cell.
(a) Only (P) and (R)
(b) Only (Q) and (R)
(c) Only (P) and (S)
(d) Only (Q) and (S)
View Solution
(ii) The metal complex ion that is paramagnetic is:
(Atomic number of Fe = 26, Cu = 29, Co = 27 and Ni = 28)
(a) [Fe(CN)4]2-
(b) [Co(NH3)6]3+
(c) [Ni(CN)4]2-
(d) [Cu(NH3)4]2+
View Solution
(iii) When KMnO4, is heated with acidified oxalic acid, gas bubbles are evolved. These gas bubbles are evolved due to the formation of:
(a) SO2 (b) CO2 (c) SO3 (d) O2
View Solution
(iv) The reaction of ethanamide with alcoholic sodium hydroxide and bromine gives:
(a) ethylamine
(b) methylamine
(c) propylamine
(d) aniline
View Solution
(v) An equimolar solution of non-volatile solutes A and B, shows a depression in freezing point in the ratio of 2:1. If A remains in its normal state in the solution, the state of B in the solution will be:
(a) normal
(b) hydrolysed
(c) associated
(d) dissociated
View Solution
(vi) Assertion: Specific conductivity of all electrolytes decreases on dilution. Reason: On dilution, the number of ions per unit volume decreases.
(a) Both Assertion and Reason are true and Reason is the cotrect explanation for Assertion
(b) Both Assertion and Reason are true but Reason is not the correct explanation for Assertion
(c) Assertion is true but Reason is false
(d) Assertion is false but Reason is true
View Solution
(vii) Assertion: Ammonolysis of alkyl halides involves the reaction between alkyl halides and alcoholic ammonia
Reason: Ammonolysis of alkyl halides produces secondary amines only.
(a) Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
(b) Both Assertion and Reason are true but Reason is not the correct explanation for Assertion
(c) Assertion is true but Reason is false
(d) Assertion is false but Reason is true
View Solution
(C) Read the passage given below and answer the questions that follow
When two solutions are separated by a semi-permeable membrane, the solvent molecules move from a solution of lower molar concentration to a solution of higher molar concentration through osmosis.
(i) Samar removed the outer hard shell of two different eggs while cooking at home. He then placed one egg in pure water and the other egg in saturated solution of sucrose. What change is he likely to observe in the eggs after few hours?
(ii) Which solution, hypertonic or hypotonic, has a higher amount of solute in same quantity of solution?
(iii) A 5% aqueous solution of glucose (molar mass = 180 g mol-1) is isotonic with 1.66% aqueous solution of urea. Calculate the molar mass of urea.
View Solution
SECTION B
Question 2
(i) Write a chemical test to distinguish between ethanol and phenol.
(ii) Give a chemical reaction to convert acetaldehyde into secondary propyl alcohol.
View Solution
Question
3
Give a reason for each of the following.
(i) Zinc, cadmium and mercury are considered as d-block elements but not regarded as transition elements.
(ii) Transition metals possess a great tendency to form complex compounds.
View Solution
Question
4
Convert the following by giving chemical equations for each
(i) Ethyl bromide to diethyl ether
(ii) Phenol to salicylaldehyde
View Solution
Question
5
Account for each of the following
(i) Zirconium (Zr) and Hafnium (Hf) are difficult to separate
(ii) Salts of Cupric (Cu2+) ion are coloured whereas salts of Cuprous (Cu+) ion are colourless.
View Solution
Question
6
How will you bring the following conversions?
(i) Benzene to biphenyl
(ii) Iodoform to acetylene
View Solution
Question
7
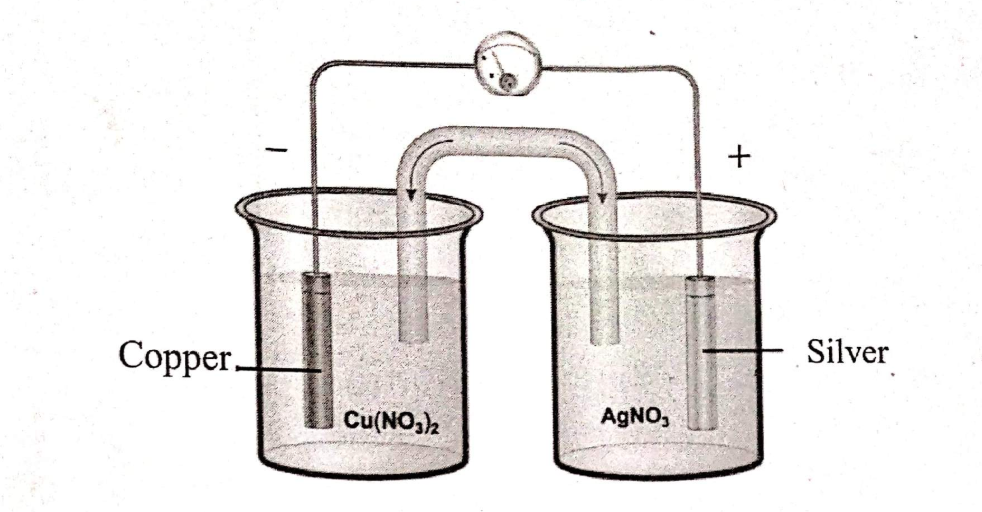
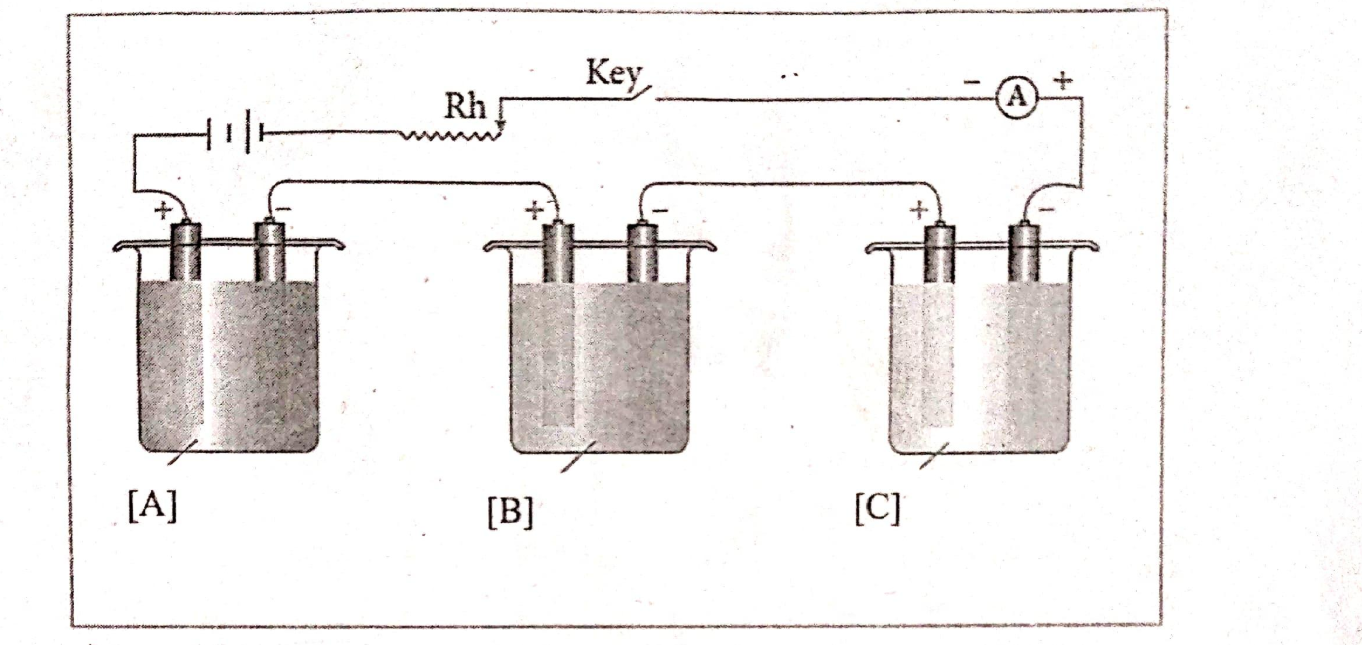
Calculate the maximum possible electrical work that can be obtained from a galvanic cell under standard conditions at 298 K.
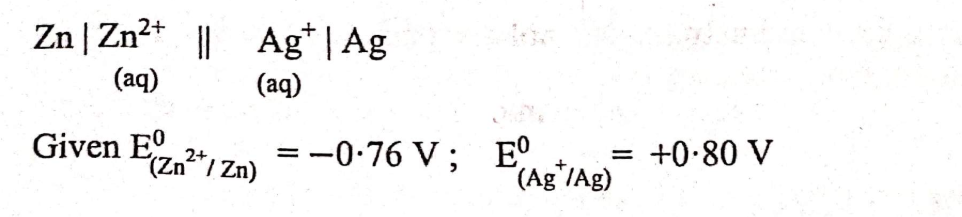
View Solution
Question
8
(i) Give a reason for each of the following
(a) Ethoxy ethane does not react with sodium, but ethanol does
(b) Ethoxy ethane with conc. HI at 373K gives C2HsOH and CHs3I but not CH30H and C2H5I.
OR
(ii) An organic compound [A] having molecular formula C4H10O forms a compound [B] with molecular formula C4H8O on oxidation. Compound [B] gives a positive iodoform test. The reaction of compound [B] with CH3MgBr followed by hydrolysis gives compound [C] with molecular formula C5H120.
Identify the compounds [A], [B] and [C]. Write the reaction for the conversion of compound [A] to compound [B].
View Solution
Question
9
If 200 cm3 of an aqueous solution of a protein contains 1.26 g of protein, the osmotic pressure of the solution at 300K is found to be 2.57 x 10-3 atm.
Calculate the molar mass of protein.
(R = 0-0821 L atm K-1 mol-1)
View Solution
Question
10
(i) Benzaldehyde is less reactive than propionaldehyde. Why?
(ii) In the preparation of ethanal by the oxidation of ethanol, ethanal should be removed immediately as it is formed. Why?
View Solution
Question
11
(i) Why is Mn+2 ion more stable than Fe+2 ion?
(Atomic number of Mn = 25 and Fe = 26)
(ii) Trivalent Lanthanoid ions such as La3+ (Z = 57) and Lu3+ (Z = 71) do not show any colour in their solution. Give a reason.
View Solution
SECTION C
Question
12
For the reaction A+ B ⇌ Product, following data was obtained:
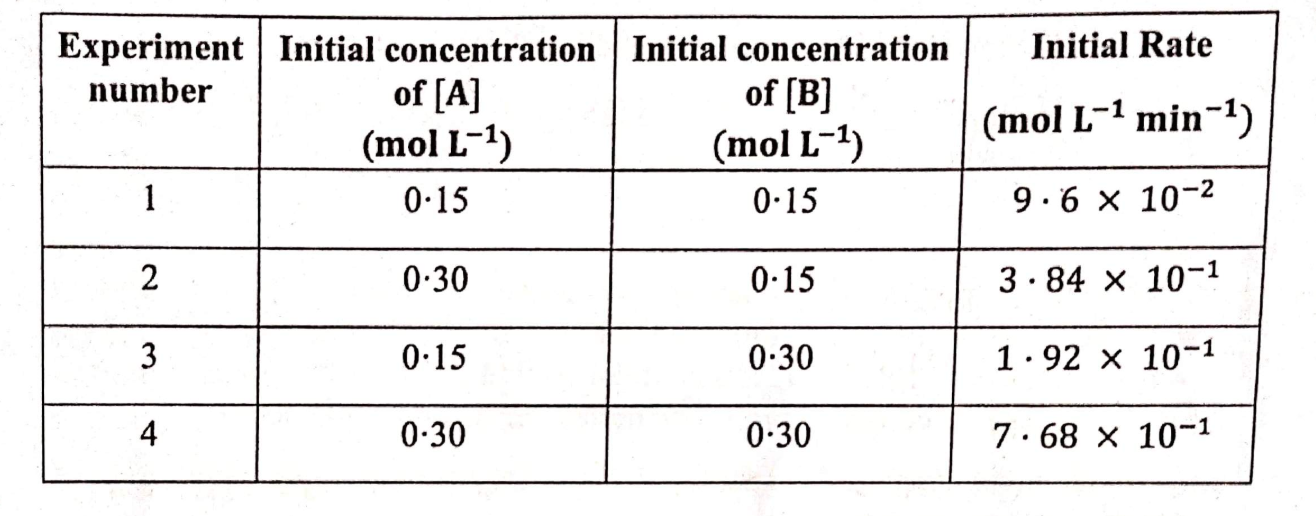
Calculate the following:
(i) The overall order of the reaction
(ii) The rate law equation
(iii) The value of rate constant
View Solution
Question
13
(i) Illustrate the following reactions by giving one suitable example in each case. (a) Coupling reaction
(b) Acetylation of ethylamine
(ii) Aniline does not give Friedel-Crafts reaction. Give a reason.
View Solution
Question
14
(i) Aradhana visits a physician as she is suffering from rickets and joint pain. Which fat-soluble vitamin should the physician prescribe to her?
(ii) Somesh put few drops of vinegar in milk. What change do you think he observed in the milk after some time? What is this phenomenon known as?
(iii) Name the product of hydrolysis of sucrose. Is it a reducing sugar or a non-reducing sugar?
View Solution
Question
15
An aqueous solution containing 12.50 g of barium chloride in 1000g of water boils at 373.0834 K. Calculate the degree of dissociation of barium chloride. Given KB for H20 = 0.52 K kg mol-1 molecular mass of BaCl2 = 208.34 g mol-1
View Solution
Question
16
An organic compound C2H4O gives red precipitate when heated with Fehling solution. It also undergoes aldol condensation in the presence of dilute NaOH.
(i) Identify the organic compound and write its IUPAC name
(ii) Which compound will be formed when this organic compound reacts with hydroxylamine
(iii) What is observed when the compound, referred to in subpart (i), is heated with ammonical silver nitrate?
View Solution
Question
17
(i) Identify the compounds [A], [B] and [C] in each of the following reactions.
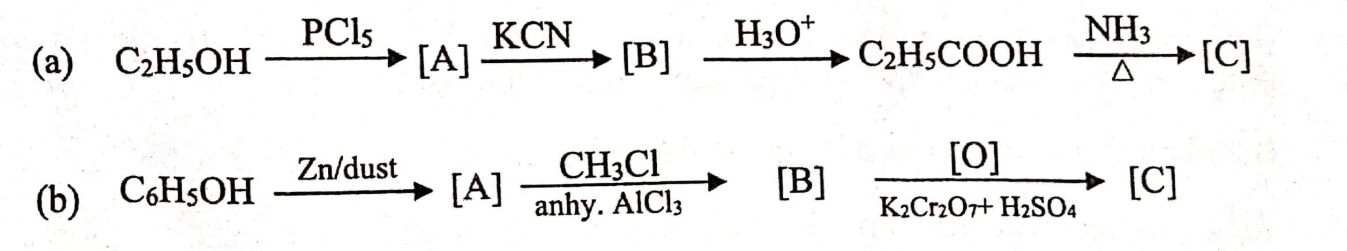
OR
(ii) Give a chemical test to distinguish between the following pairs of compounds.
(a) Ethanol and methanol
(b) Ethanol and Ethanal
(c) Propan-2-ol and 2-methyl propan-2-ol
View Solution
Question
18
(i) The rate constant of a reaction at 5OOK and 700K are 0.02 sec-1 and 0.07 sec-1 respectively. Calculate the value of Ea (activation energy)
(ii) A radioactive substance which emits alpha particle follows first order reaction.The half-life period of this radioactive substance is 30 hours. Calculate the fraction in percent(%) of the radioactive substance which remains after 90 hours.
View Solution
SECTION D
Question
19
(i) An organic compound [A], having a specific smell forms two compounds [B] and [C] by reacting with conc. sodium hydroxide. The molecular formula of compound [B] is C7H8O, which forms compound [A] again on oxidation. Compound [C] forms benzene on heating with soda lime.
Write the structures of compounds [A], [B] and [C]. Also, write the reactions involved.
(ii) Identify the compounds [A] and [B] in the reactions given below:
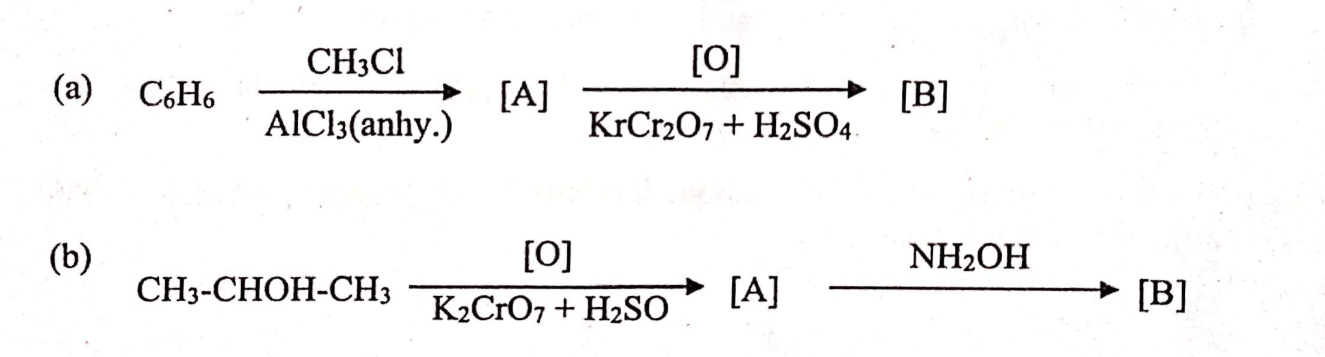
View Solution
Question
20
(i) Acoordination compound has a formula CoCl3.4NH3. It precipitates silver ions as AgCl and its molar conductance corresponds to a total of two ions
Based on this information, answer the following questions
(a) Deduce structural formula of the complex compound.
(b) Write the IUPAC name of the complex compound.
(c) Draw the geometrical isomers of the complex compound.
(ii) Give a chemical test to show that [Co(NH3)5Cl]SO4
[Co(NH3)5SO4]Cl are ionisation isomers.
View Solution
Question
21