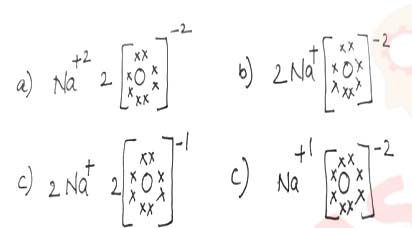This question paper comprises 39 questions. All questions are compulsory.
This question paper is divided into five sections- A, B, C, D and E.
Section A -
Questions No. 1 to 20 are multiple choice questions. Each question
carries 1 mark.
Section B -
Questions No. 21 to 26 are very short answer type questions. Each
question carries 2 marks. Answer to these questions should be in the range of
30 to 50 words.
Section C -
Questions No. 27 to 33 are short answer type questions. Each
question carries 3 marks. Answer to these questions should in the range of
50 to 80 words.
Section D -
Questions No. 34 to 36 are long answer type questions. Each
question carries 5 marks.
Answer to these questions should be in the range of
80 to 120 words.
Section E -
Questions No. 37 to 39 are of 3 source-based / case-based units of
assessment carrying 4 marks each with sub-parts.
Section-A
This section has 20 multiple choice questions (Q.No. 1 -
20). All questions are
compulsory
Question
1
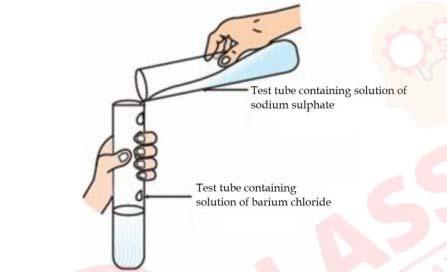
Identify the product which represents the solid state in the above reaction.
a) Barium chloride
b) Barium sulphate
c) Sodium chloride
d) Sodium sulphate
View
Solution
Question 2
The colour of the solution observed after 30 minutes of placing zinc metal to copper
sulphate solution is
a) Blue
b) Colourless
c) Dirty green
d) Reddish Brown
View
Solution
Question
3
Mild non-corrosive basic salt is
a) Ca(OH)2
b) NaCl
c) NaOH
d) NaHCO3
View
Solution
Question
4
On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless
gas is
produced when a burning match stick is brought near it. Which of the following correctly
represents metal ‘X’?
a) Sodium
b) Sulphur
c) Copper
d) Silver
View
Solution
Question
5
Which one of the following correctly represents Sodium oxide?
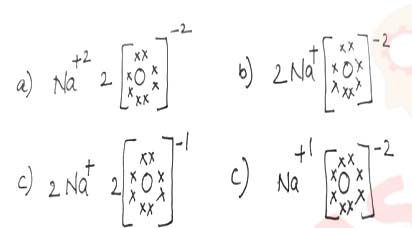
View
Solution
Question
6
An element with atomic number_____ will form a basic oxide.
a) 7 (2,5)
b) 17 (2,8,7)
c) 14 (2,8,4)
d) 11 (2,8,1)
View
Solution
Question
7
An element ‘M' has 50% of the electrons filled in the 3rd shell as in the 2nd
shell. The
atomic number of ‘M’ is:
a) 10
b) 12
c) 14
d) 18
View
Solution
Question
8
Generally food is broken and absorbed within the body of organisms. In which of
the
following organisms is it done outside the body?
a) Amoeba
b) Mushroom
c) Paramoecium
d) Lice
View
Solution
Question
9
Receptors are usually located in sense organs. Gustatory receptors are present in
a) tongue
b) nose
c) eye
d) ear
View
Solution
Question
10
A farmer wants to grow banana plants genetically similar enough to the plants
already
available in his field. Which one of the following methods would you suggest for this
purpose?
a) Regeneration
b) Budding
c) Vegetative propagation
d) Sexual reproduction
View
Solution
Question
11
Height of a plant is regulated by:
a) DNA which is directly influenced by growth hormone.
b) Genes which regulate the proteins directly.
c) Growth hormones under the influence of the enzymes coded by a gene.
d) Growth hormones directly under the influence a gene
View
Solution
Question
12
A sportsman, after a long break of his routine exercise, suffered muscular cramps
during a
heavy exercise session. This happened due to:
a) lack of carbon dioxide and formation of pyruvate.
b) presence of oxygen and formation of ethanol.
c) lack of oxygen and formation of lactic acid.
d) lack of oxygen and formation of carbon dioxide.
View
Solution
Question
13
An object is placed in front of a convex mirror. Its image is formed :
a) at a distance equal to the object distance in front of the mirror.
b) at twice the distance of the object in front of the mirror.
c) half the distance of the object in front of the mirror.
d) behind the mirror and it’s position varies according to the object distance.
View
Solution
Question
14
When light enters the atmosphere it strikes on extremely fine particles, which
deflect the
rays of light in all possible directions, This is due to -
a) reflection of light
b) atmospheric refraction
c) scattering of light
d) dispersion of light
View
Solution
Question
15
In 1987, an agreement was formulated by the United Nations Environment Programme
(UNEP) to freeze the production of “X” to prevent depletion of “Y”. “X” and “Y”
respectively referred here are:
a) Ozone; CFCs
b) CFCs; rays UV
c) CFCs; Ozone
d) UV rays; Diatomic oxygen
View
Solution
Question
16
Which of the following features relates to biodegradable substances?
a) Broken down by biological processes
b) Remain inert
c) Persist in environment for long time
d) May harm the ecosystem
View
Solution
Question
17
For questions number 17 to 20, two statements are given- one labelled
as Assertion (A) and the other labelled as Reason (R). Select the correct
answer to these questions from the codes (a), (b), (c) and (d) as given
below:
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the
correct explanation of the Assertion (A).
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not
the correct explanation of the Assertion (A).
(c) Assertion (A) is true, but Reason (R) is false.
(d) Assertion (A) is false, but Reason (R) is true.
Assertion: Rusting of Iron is endothermic in nature.
Reason: As the reaction is slow, the release of heat is barely evident
View
Solution
Question
18
Assertion: Probability of survival of an organism produced through sexual
reproduction is
more than that of organism produced through asexual mode.
Reason: Variations provide advantages to individuals for survival
View
Solution
Question
19
Assertion : A compass needle is placed near a current carrying wire. The deflection
of the
compass needle decreases when the magnitude of the current in the wire is increased.
Reason : The strength of a magnetic field at a point near the conductor increases on
increasing the current.
View
Solution
Question
20
Assertion: Biodegradable substances result in the formation of compost and natural
replenishment.
Reason: It is due to breakdown of complex inorganic substances into simple organic
substances.
View
Solution
SECTION-B
Question
21
Dil. HCl is added to Zn granules.” How will you prove that chemical change has
taken place
here? Support your response with two arguments.
View
Solution
Question
22
State the post-fertilisation changes that lead to fruit formation in plants.
View
Solution
Question
23
What is the purpose of making urine in the human body? Name the organs that stores
and
releases the urine.
OR
Why do arteries have thick and elastic walls whereas veins have valves?
View
Solution
Question
24
The refractive indices of three media are given below:
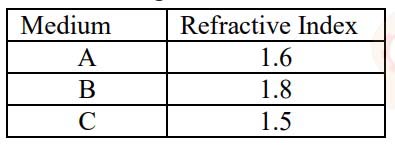
A ray of light is travelling from A to B and another ray is travelling from B to C.
(a) In which of the two cases the refracted ray bends towards the normal?
(b) In which case does the speed of light increase in the second medium?
Give reasons for your answer
View
Solution
Question
25
A piece of wire of resistance R is cut into three equal parts. These parts are then
connected
in parallel. If the equivalent resistance of this parallel combination is R1, what is the value
of the ratio R1/R
OR
Refer to the image below and state how the magnetic field pattern indicates regions where
the magnetic field is stronger outside the magnet? What happens to the magnetic field
when the current in the circuit is reversed?
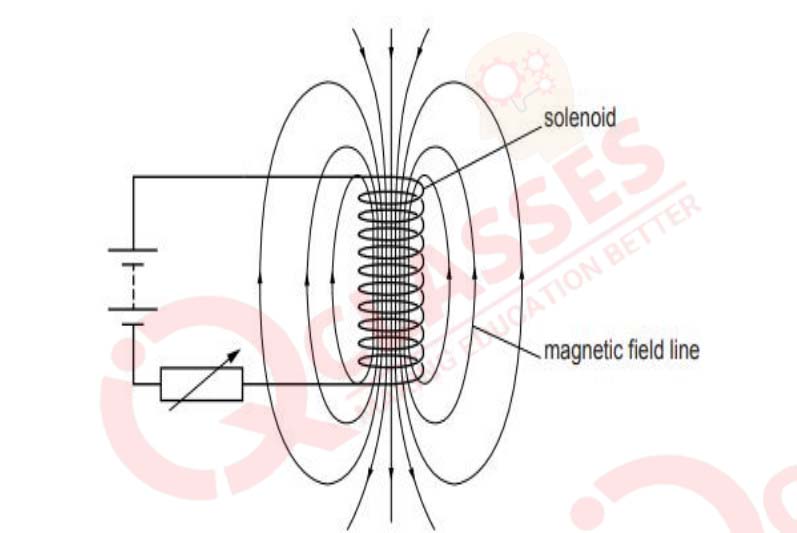
View
Solution
Question
26
Study the food chain given below and answer the questions that follow:
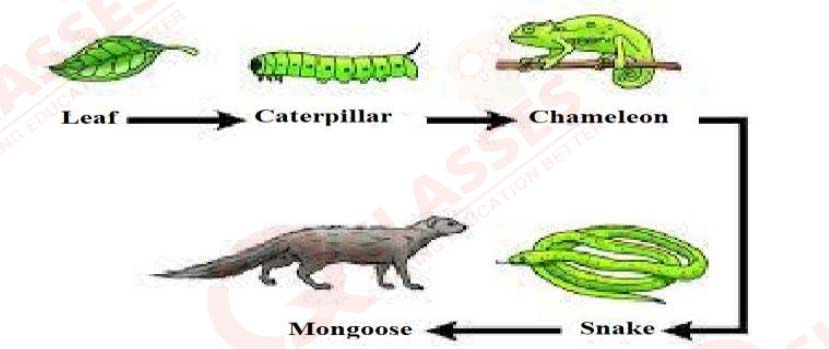
a) If the amount of energy available at the third trophic level is 100 joules, then how
much energy will be available at the producer level? Justify your answer.
b) Is it possible to have 2 more trophic levels in this food chain just before the fourth
trophic level? Justify your answer.
View
Solution
SECTION-C
Question
27
The given reaction shows one of the processes to extract the metals like Iron and
Manganese.
MnO₂ (s) + Al(s) → Mn(l) + Al₂O₃(s) + Heat
a) Give reason why the above reaction is known as a thermite reaction.
b) Identify the substance oxidised and reduced in the above reaction.
c) Give a reason why Aluminium is preferably used in thermite reactions.
View
Solution
Question
28
An element ‘M’ with electronic configuration 2 8 3 combines separately with Cl-
, SO4-2
anions. Write the chemical formulae of the compounds formed. Predict with the suitable
reason the nature of the bond formed by element ‘M’ in general. How will the electrical
conductivity of the compounds formed vary with respect to ‘M’?
OR
A reddish-brown metal ‘X’, when heated in air, gives a black compound ‘Y’, which when
heated in presence of H₂ gas gives ‘X’ back. ‘X’ is refined by the process of electrolysis;
this refined form of ‘X’ is used in electrical wiring.
Identify ‘X’ and ‘Y’. Draw a well-labeled diagram to represent the process of refining ‘X’
View
Solution
Question
29
We are advised to take iodised salt in our diet by doctors. Justify it's importance
in our
body
View
Solution
Question
30
What is the probability of a girl or a boy being born in a family? Justify your
answer.
View
Solution
Question
31
(i) Explain why the refractive index of any material with respect to air is always
greater 1.
(ii) In the figure below a light ray travels from air into the semi-circular plastic block. Give
a reason why the ray does not deviate at the semi-circular boundary of the plastic
block
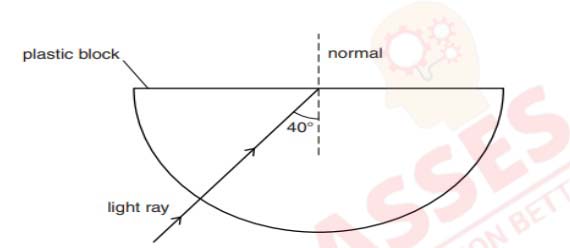
(iii) Complete the ray diagram of the above scenario when the light ray comes out of the
plastic block from the top flat end.
View
Solution
Question
32
(i) State the law that explains the heating effect of current with respect to the
measurable
properties in an electrical circuit.
(ii) List the factors on which the resistance of a conductor depends.
View
Solution
SECTION-D
Question
33
Anannya responded to the question: Why do electrical appliances with metallic
bodies are
connected to the mains through a three pin plug, whereas an electric bulb can be connected
with a two pin plug?
She wrote: Three pin connections reduce heating of connecting wires.
(i) Is her answer correct or incorrect? Justify.
(ii) What is the function of a fuse in a domestic circuit?
View
Solution
Question
34
a) Rehmat classified the reaction between Methane and Chlorine in presence of
sunlight as
a substitution reaction. Support Rehmat’s view with suitable justification and illustrate
the reaction with the help of a balanced chemical equation.
b) Chlorine gas was prepared using electrolysis of brine solution. Write the chemical
equation to represent the change. Identify the other products formed in the process and
give one application of each.
OR
Raina while doing certain reactions observed that heating of substance ‘X’ with vinegar like
smell with a substance ‘Y’ (which is used as an industrial solvent) in presence of conc.
Sulphuric acid on a water bath gives a sweet-smelling liquid ‘Z’ having molecular formula
C4H8O2. When heated with caustic soda (NaOH), ‘Z’ gives back the sodium salt of and the
compound ‘Y’.
Identify ‘X’, ‘Y’, and ‘Z’. Illustrate the changes with the help of suitable chemical
equations.
View
Solution
Question
35
Given below are certain situations. Analyze and describe its possible impact on a
person:
a) Testes of a male boy are not able to descend into scrotum during his embryonic
development.
b) Vas deferens of a man is plugged.
c) Prostate and seminal vesicles are not functional.
d) Egg is not fertilised in a human female.
e) Placenta does not attach to the uterus optimally.
OR
a) A doctor has advised Sameer to reduce sugar intake in his diet and do regular exercise
after checking his blood test reports. Which disease do you think Sameer is suffering
from? Name the hormone responsible for this disease and the organ producing the
hormone.
b) Which hormone is present in the areas of rapid cell division in a plant and which
hormone inhibits the growth?
View
Solution
Question
36
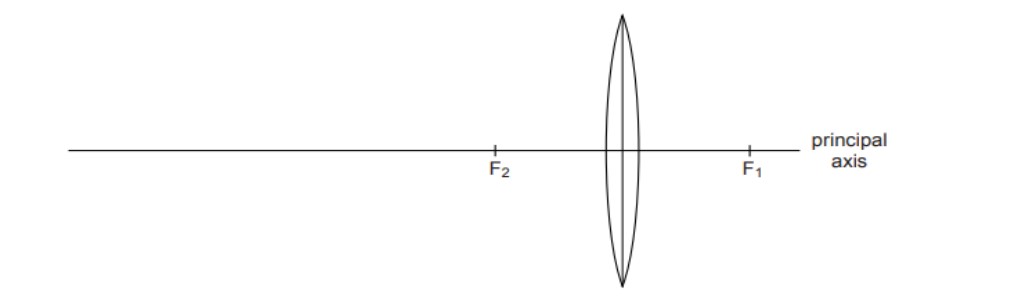
The above image shows a thin lens of focal length 5m.
(i) What is the kind of lens shown in the above figure?
(ii) If a real inverted image is to be formed by this lens at a distance of 7m from the optical
centre, then show with calculation where should the object be placed?
(iii) Draw a neatly labelled diagram of the image formation mentioned in (ii)
OR
A 10 cm long pencil is placed 5 cm in front of a concave mirror having a radius of curvature
of 40 cm
(i) Determine the position of the image formed by this mirror.
(ii) What is the size of the image?
(iii)Draw a ray diagram to show the formation of the image as mentioned in the part (i).
View
Solution
SECTION-E
The following questions are source-based/ case-based questions. Read the case
carefully and answer the questions that follow.
Question
37
a) Rehmat classified the reaction between Methane and Chlorine in presence of
sunlight as
a substitution reaction. Support Rehmat’s view with suitable justification and illustrate
the reaction with the help of a balanced chemical equation.
b) Chlorine gas was prepared using electrolysis of brine solution. Write the chemical
equation to represent the change. Identify the other products formed in the process and
give one application of each.
OR
Raina while doing certain reactions observed that heating of substance ‘X’ with vinegar like
smell with a substance ‘Y’ (which is used as an industrial solvent) in presence of conc.
Sulphuric acid on a water bath gives a sweet-smelling liquid ‘Z’ having molecular formula
C4H8O2. When heated with caustic soda (NaOH), ‘Z’ gives back the sodium salt of and the
compound ‘Y’.
Identify ‘X’, ‘Y’, and ‘Z’. Illustrate the changes with the help of suitable chemical
equations.
View
Solution
Question
38
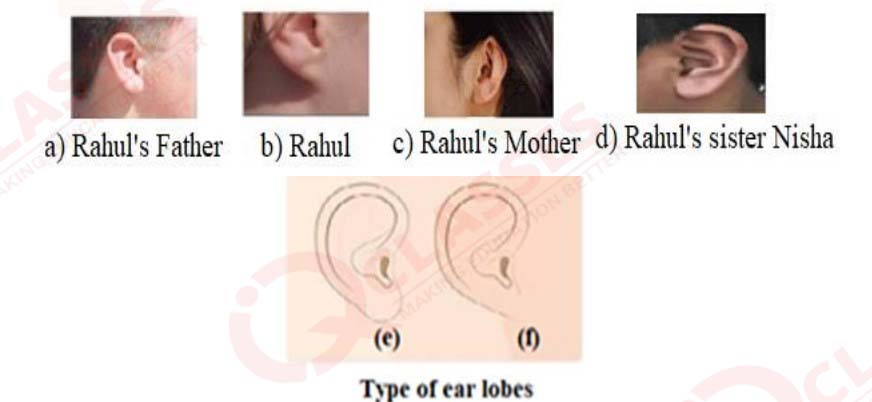
Figures (a) to (d) given below represent the type of ear lobes present in a family
consisting
of 2 children – Rahul, Nisha and their parents.
Excited by his observation of different types of ear lobes present in his family, Rahul
conducted a survey of the type of ear lobes found {Figure (e) and (f)} in his classmates. He
found two types of ear lobes in his classmates as per the frequency given below:
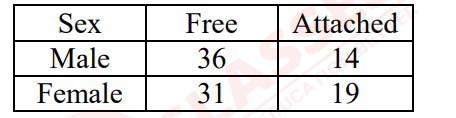
On the basis of above data answer the following questions.
a) Which of the two characteristics - ‘free ear lobe’ or ‘attached ear lobe’ appears to be
dominant in this case? Why?
b) Is the inheritance of the free ear lobe linked with sex of the individual? Give reason for
your answer.
c) What type of ear lobe is present in father, mother, Rahul and his sister Nisha? Write the
genetic constitution of each of these family members which explains the inheritance of
this character in this family?
(Gene for Free ear lobe is represented by F and gene for attached ear lobe is represented
by f for writing the genetic constitution).
OR
Suresh’s parents have attached earl obes. What type of ear lobe can be seen in Suresh
and his sister Siya? Explain by giving the genetic composition of all.
View
Solution
Question
39
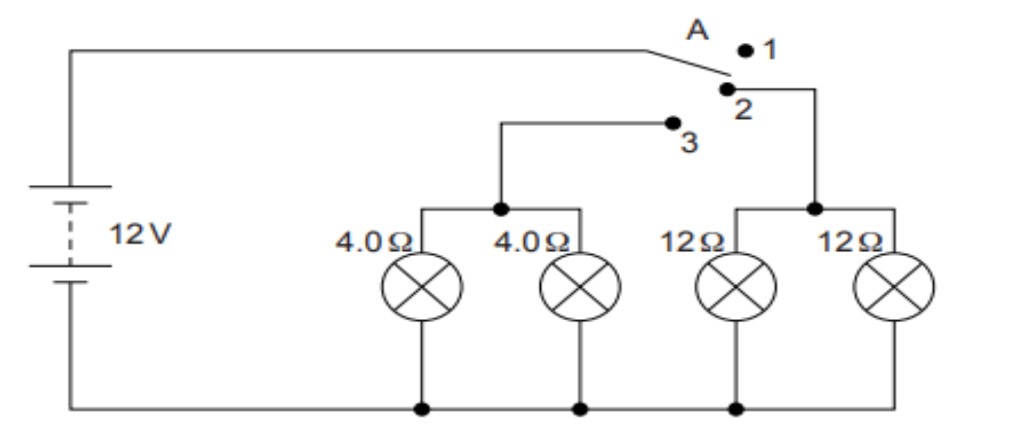
Vinita and Ahmed demonstrated a circuit that operates the two headlights and the two
sidelights of a car, in their school exhibition. Based on their demonstrated circuit, answer
the following questions.
(i) State what happens when switch A is connected to
a) Position 2
b) Position 3
(ii) Find the potential difference across each lamp when lit.
(iii) Calculate the current
a) in each 12 Ω lamp when lit.
b) In each 4 Ω lamp when lit.
OR
(iv) Show, with calculations, which type of lamp, 4.0 Ω or 12 Ω, has the higher power
View
Solution