Acid, Bases & Salts Chapter Board Questions Class 10 CBSE
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Acid, Bases & Salts. These important notes,board questions and predicted questions are based on CBSE. board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
Baking soda is a mixture of: (i) Sodium carbonate and acetic acid
(ii) Sodium carbonate and tartaric acid
(iii) Sodium hydrogen carbonate and tartaric acid
(iii) Sodium hydrogen carbonate and acetic acid
solutions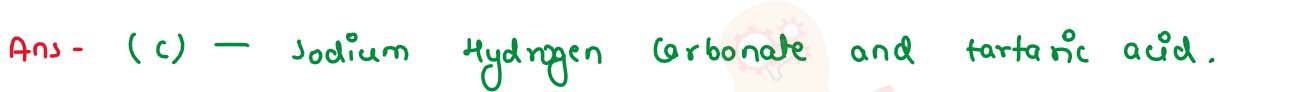
(ii) Sodium carbonate and tartaric acid
(iii) Sodium hydrogen carbonate and tartaric acid
(iii) Sodium hydrogen carbonate and acetic acid
solutions
Q2
The chemical formula for plaster of paris is :
(a) CaSO4.2H2O
(b) CaSO4. H2O
(c) CaSO4. 1/2 H2O
(d) 2 CaSO4. H2O
solutions
(a) CaSO4.2H2O
(b) CaSO4. H2O
(c) CaSO4. 1/2 H2O
(d) 2 CaSO4. H2O
solutions

Q3
How is washing soda prepared from sodium carbonate? Give its chemical equation. State the type of
this salt. Name the type of hardness of water which can be removed by it?
solutions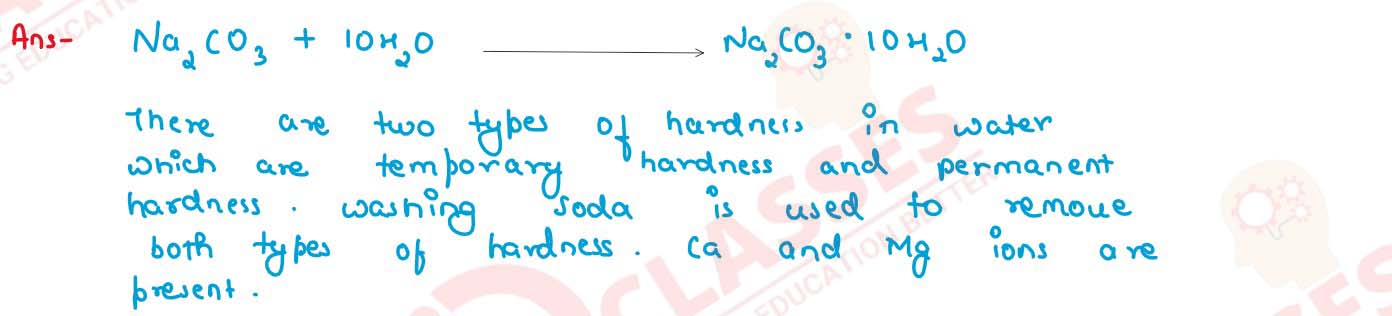
solutions

2019
Q4
Difference between an acid and a base. With the help of suitable examples explain the term
neutralization and the formation of -
(i) acidic ,
(ii) basic and
(iii) neutral salts
solutions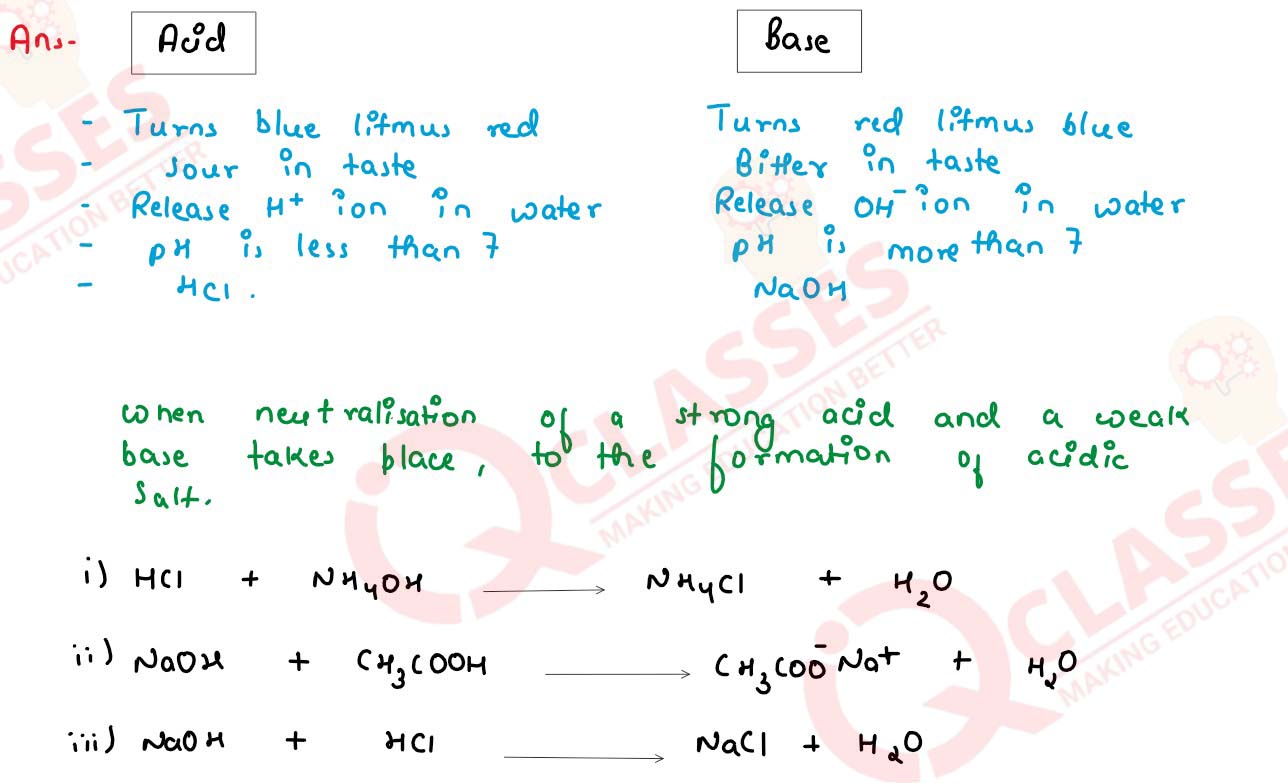
(i) acidic ,
(ii) basic and
(iii) neutral salts
solutions

2018
Q5
2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test
tube. When the contents are warmed, a gas evolves which is bubbled through a soap solution before
testing. Write the equation of the chemical I reaction involved and the test to detect the gas. Name
the gas which will be evolved when the same metal reacts With dilute solution of a strong acid.
OR
The pH of salt used to make tasty and crispy pakoras is 14. Identify the salt and write a chemical equation for its formation. List its 2 uses.
solutions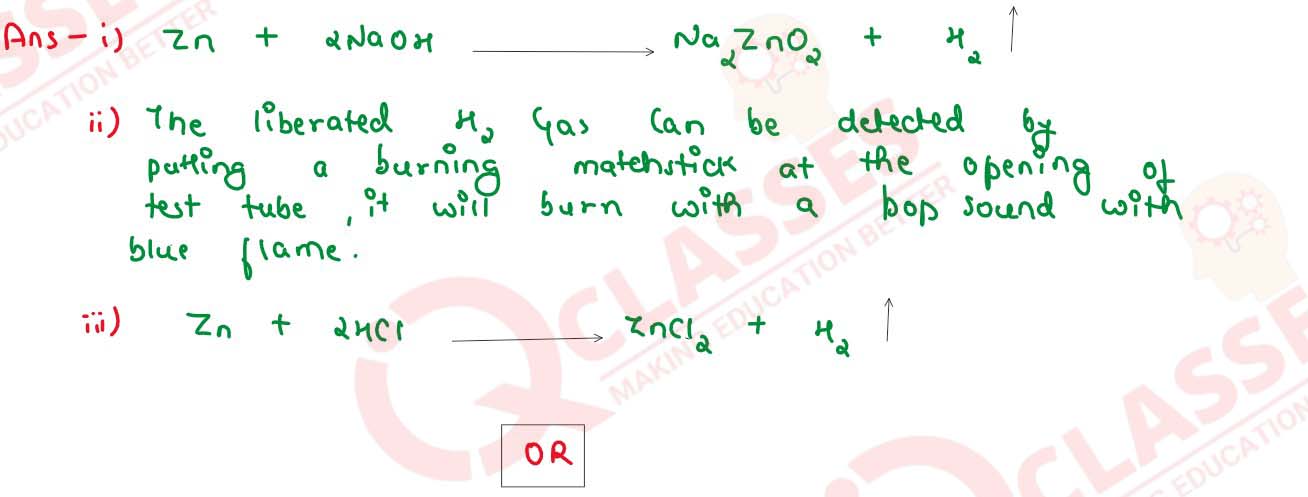
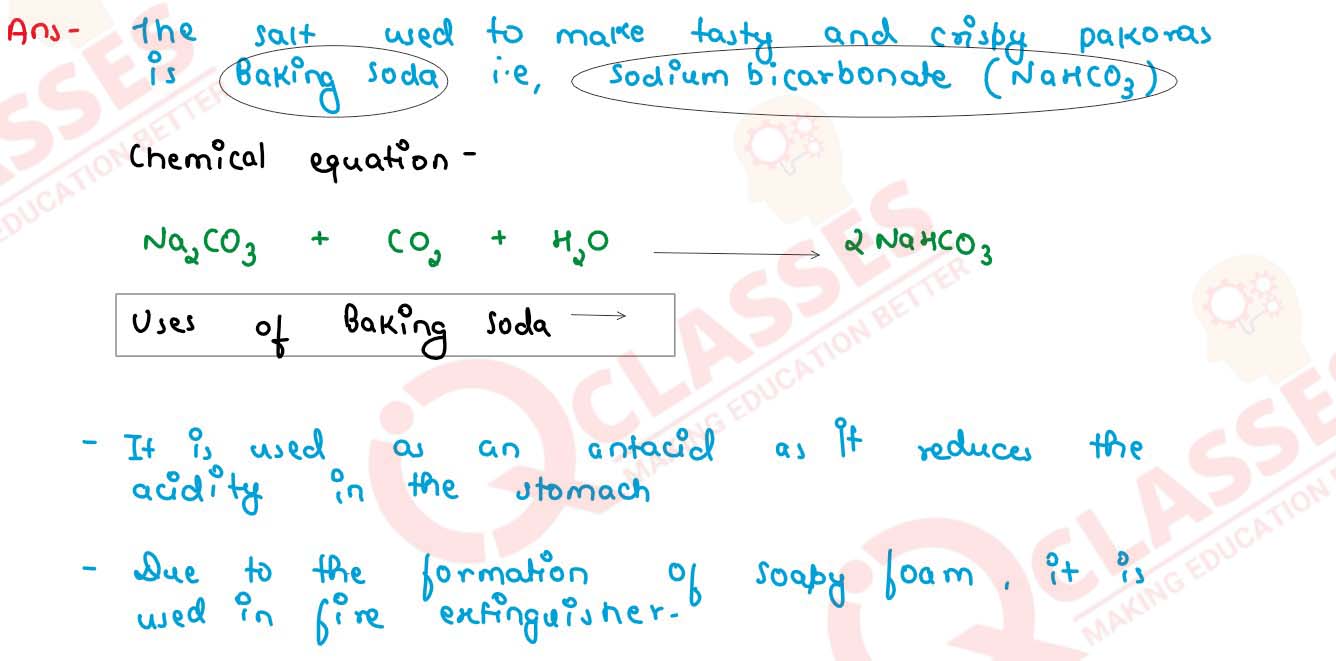
The pH of salt used to make tasty and crispy pakoras is 14. Identify the salt and write a chemical equation for its formation. List its 2 uses.
solutions


2017
Q6
When you add a few drops of acetic acid to a test tube containing sodium bicarbonate powder what
will you observe ?
solutions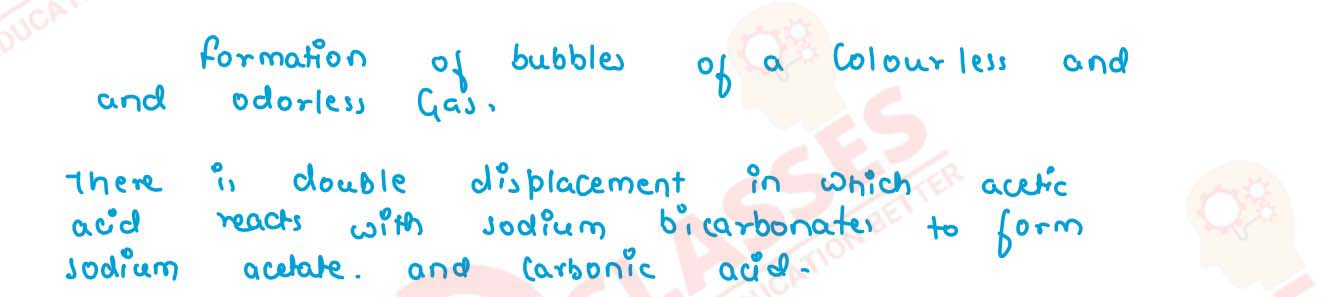
solutions

Q7
A student require hard water for an experiment In his laboratory which is not available in the
neighbouring area. In the laboratory there are some salts, which when dissolved in distilled water
can convert it into hard water. Select from the following groups of salts, a group, each salt of
which when dissolved in distilled water will make it hard
(A) Sodium chloride, Potassium chloride
(B) Sodium sulphate, Potassium sulphate
(C) Sodium sulphate, Calcium sulphate
(D) Calcium sulphate, Calcium chloride
solutions
(A) Sodium chloride, Potassium chloride
(B) Sodium sulphate, Potassium sulphate
(C) Sodium sulphate, Calcium sulphate
(D) Calcium sulphate, Calcium chloride
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment