Calorimetry Chapter Board Questions Class10 ICSE
Here we provide Class 10 Maths important notes,board questions and predicted questions with Answers for chapter Calorimetry .These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 Maths syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
(i) Define heat capacity of a substance.
(ii) Write the Sl unit of heat capacity.
(iii) What is the relationship between heat capacity and heat capacity of a substance?
solutions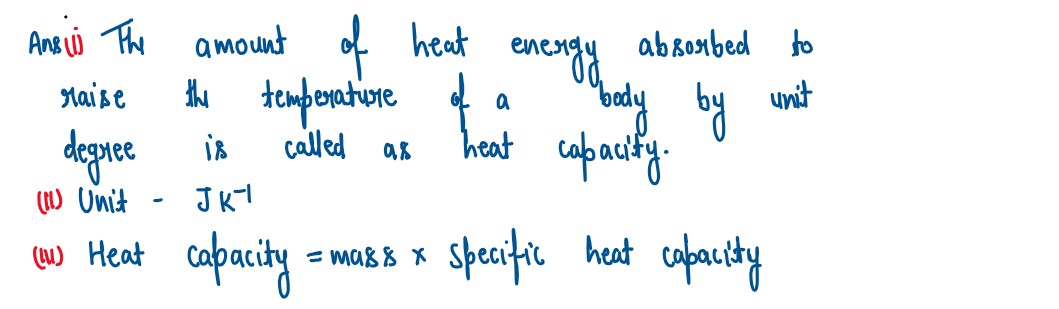
(ii) Write the Sl unit of heat capacity.
(iii) What is the relationship between heat capacity and heat capacity of a substance?
solutions
Q2
(b)
The diagram below shows the change f phases of a substance on a temperature vs
time graph on heating the substance at a constant rate.
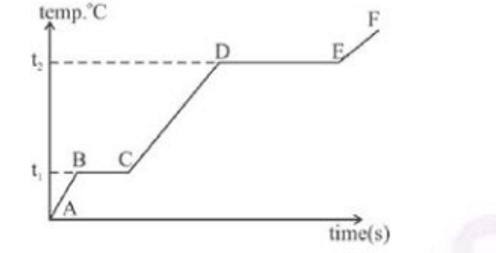
(i) Why is the slope of CD less than slope of AB?
(ii) What is the boiling and melting point ofthe substance?
solutions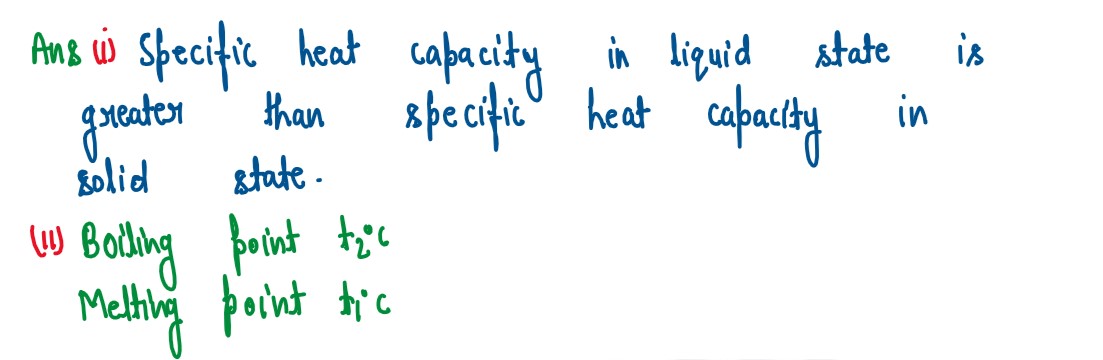

(i) Why is the slope of CD less than slope of AB?
(ii) What is the boiling and melting point ofthe substance?
solutions
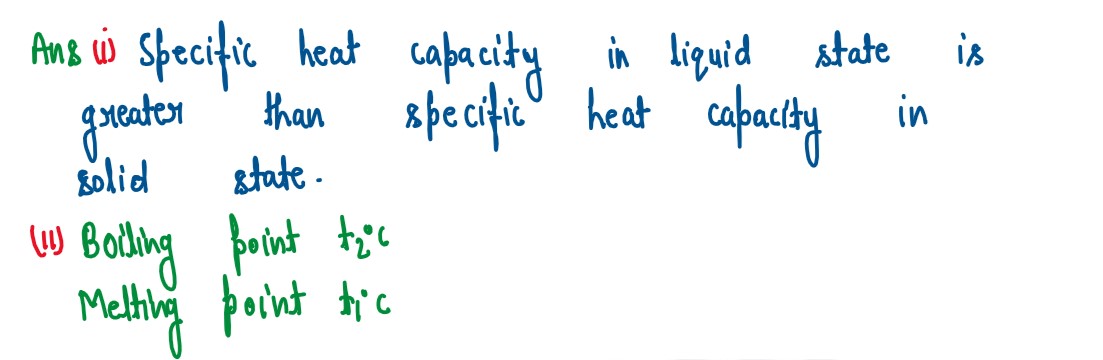
Q3
A piece of ice of mass 60 g is dropped into 140 g of water at 50°C
Calculate the final temperature of water When all the ice has melted.
(Assume no heat is lost to the surrounding)
Specific heat capacity of water — 4.2 Jg-1k-1
Specific latent heat of fusion of ice — 336 Jg-1
solutions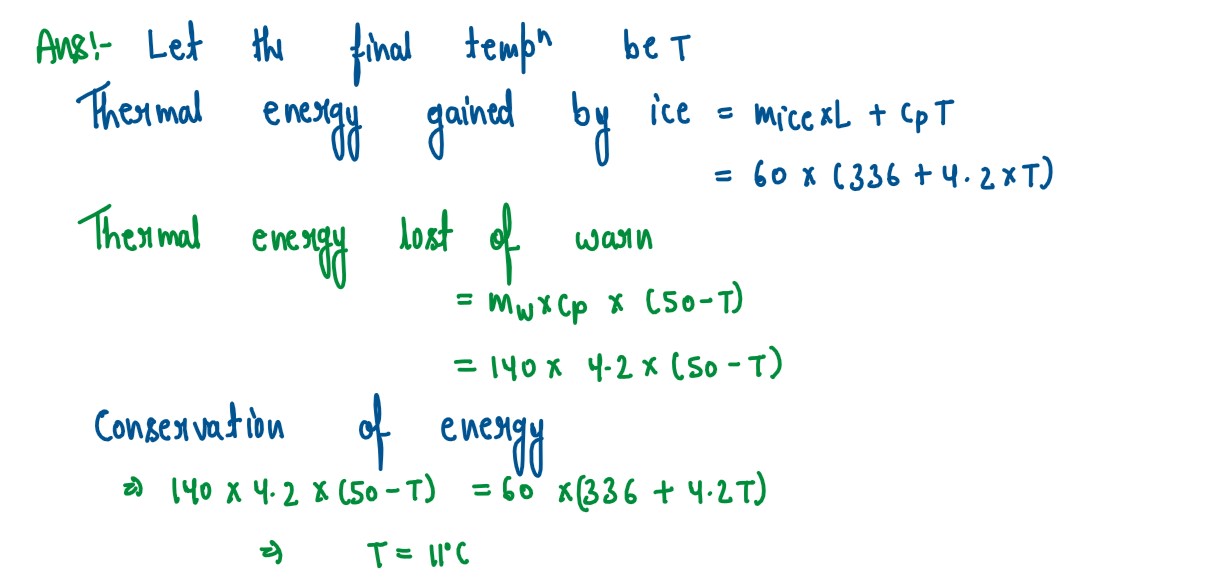
Calculate the final temperature of water When all the ice has melted.
(Assume no heat is lost to the surrounding)
Specific heat capacity of water — 4.2 Jg-1k-1
Specific latent heat of fusion of ice — 336 Jg-1
solutions
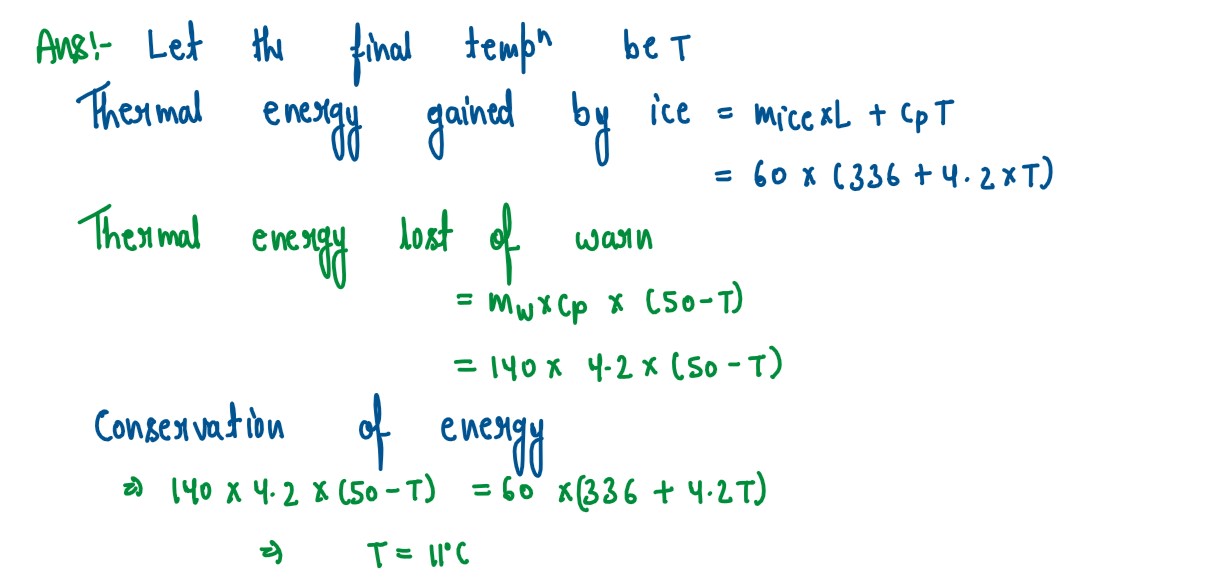
2019
Q1
(i)Name the physical quantity which is measured in calories
(ii)How is the calorie related to the S.I unit of that quantity
solutions
(ii)How is the calorie related to the S.I unit of that quantity
solutions

Q2
Thc specific heat capacity ofa substance A is 3,800 Jkg-1K-1 and that of a
substance
B
is 400 Jkg-1K-1. Which of the two substances is a good conductor of heat? Give
a reason
for your answer.
solutions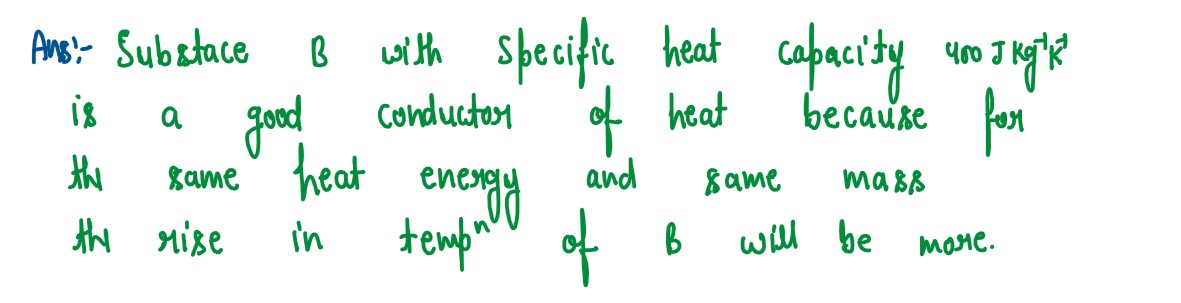
solutions
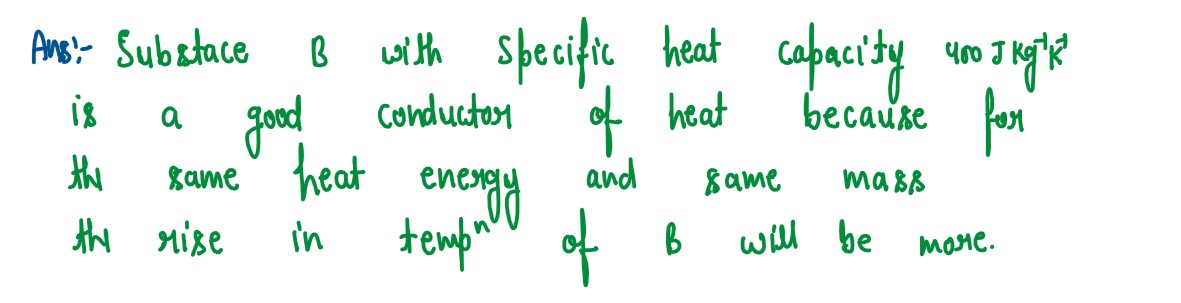
Q3
(i) State whether the specific heat capacity of a substance remains the same when
its state changes from solid to liquid.
(ii) Give one example to support your answer.
solutions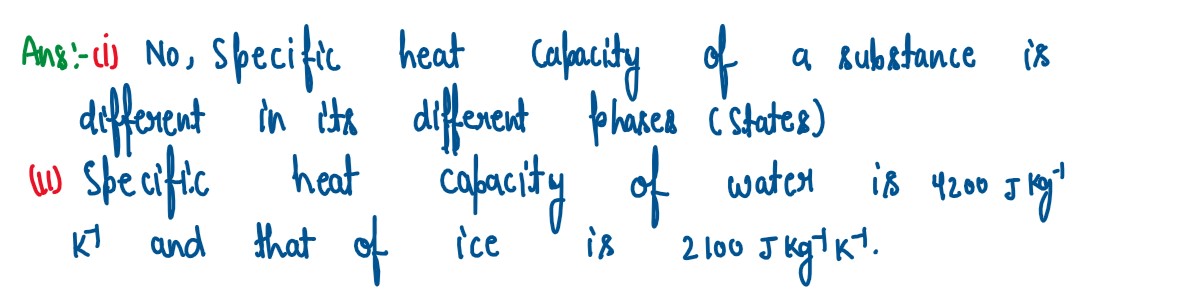
(ii) Give one example to support your answer.
solutions

Q4
(i) Define Calorimetry.
(ii) Name the material used for making a Calorimeter.
(iii) Why is a Calorimeter made up of thin sheets of the above material answered in
solutions

(ii) Name the material used for making a Calorimeter.
(iii) Why is a Calorimeter made up of thin sheets of the above material answered in
solutions


Q5
The melting of naphthalene is 80°C and the room temperature is 30°C. A
sample of liquid naphthalene at 100°C is cooled down to the room temperature. Draw
a tenwature time graph to represent this cooling. In the graph. mark the region
which corresjx»nds to the freezing process.
solutions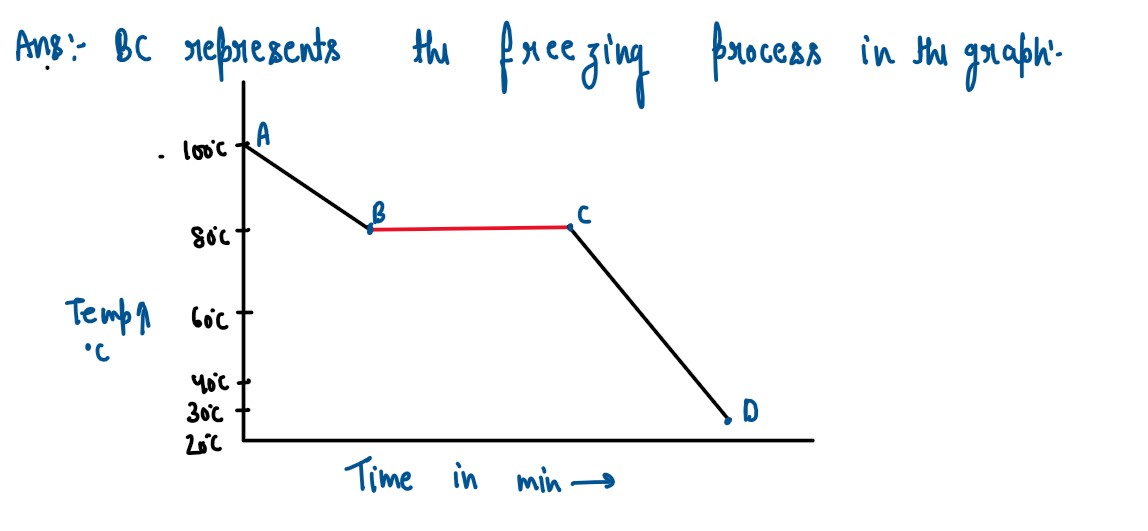
solutions

Q6
104 g ofwater at 30°C is taken in a calorimeter made of copper of mass 42 g. When
a certain mass of ice at O°C is added to it, the final steady tenFature of the mixture
aner the ice has melted, was found to be 10°C. Find the mass of ice added. [Specific
heat capacity of water — 4.2 Jg-1°C-1, latent heat of fusion of ice 336
Jg-1
Specific heat capacity of = 0.4 Jg-1°C-1
solutions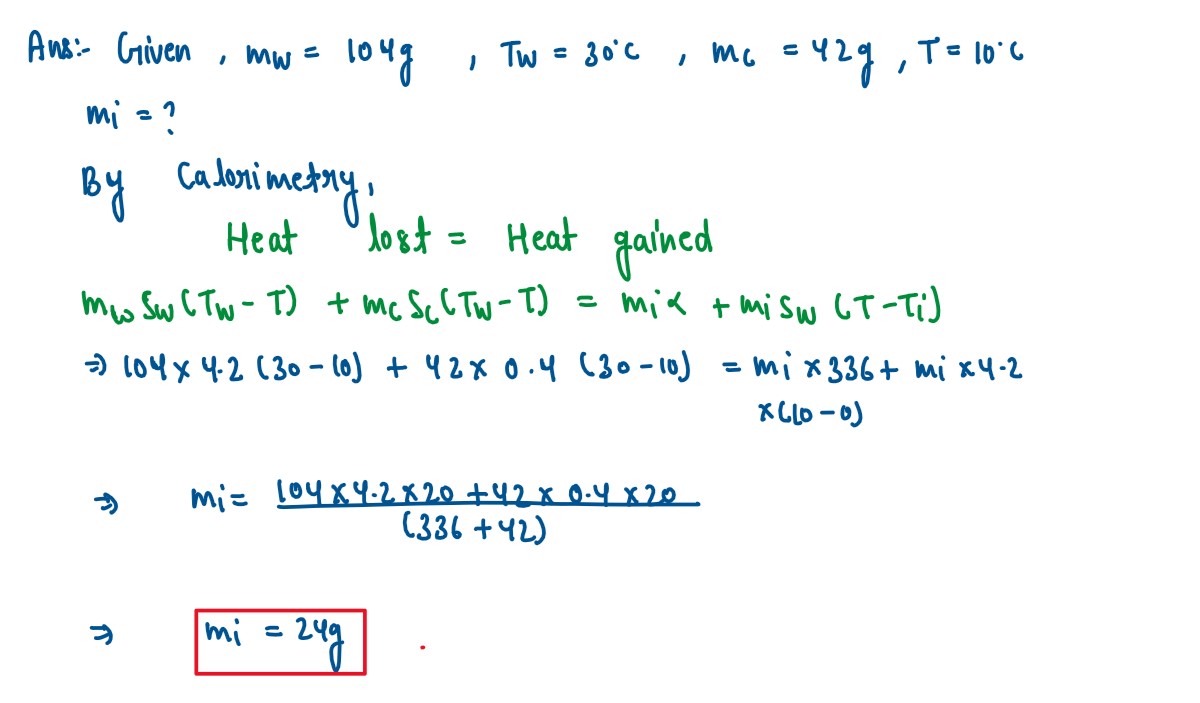
solutions

2018
Q1
(i) How can a temlrrature in degree Celsius be converted into S.I. unit of temperature
(ii) A liquid X has the maximum specific heat capacity and is used as a coolant in
Car radiators. Name the liquid X.
solutions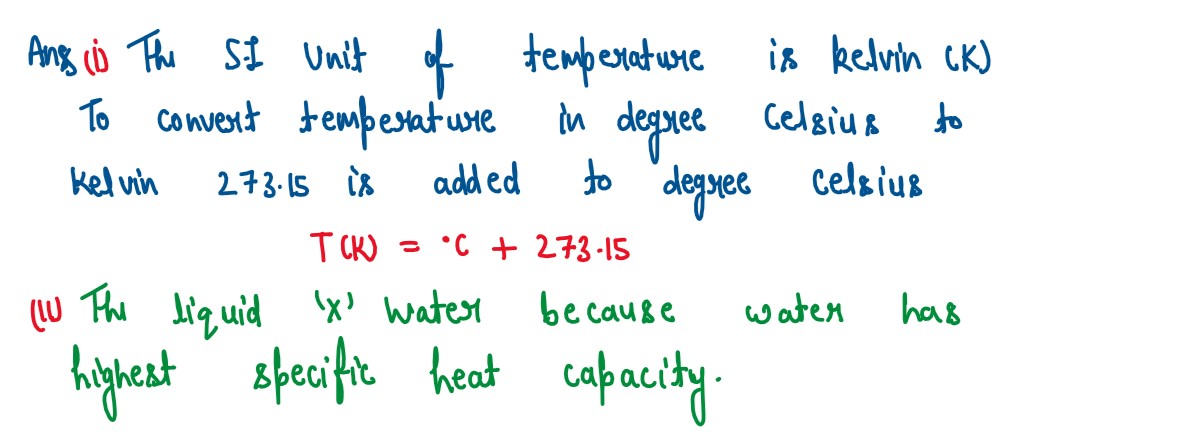
solutions
Q2
A solid metal weighing 150 g melts at its melting point of 800 °C by providing heat
at the rate of 100 W. The time taken for it to completely melt at the same temperature
is 4 min. What is the specific latent heat of fusion of the metal?
solutions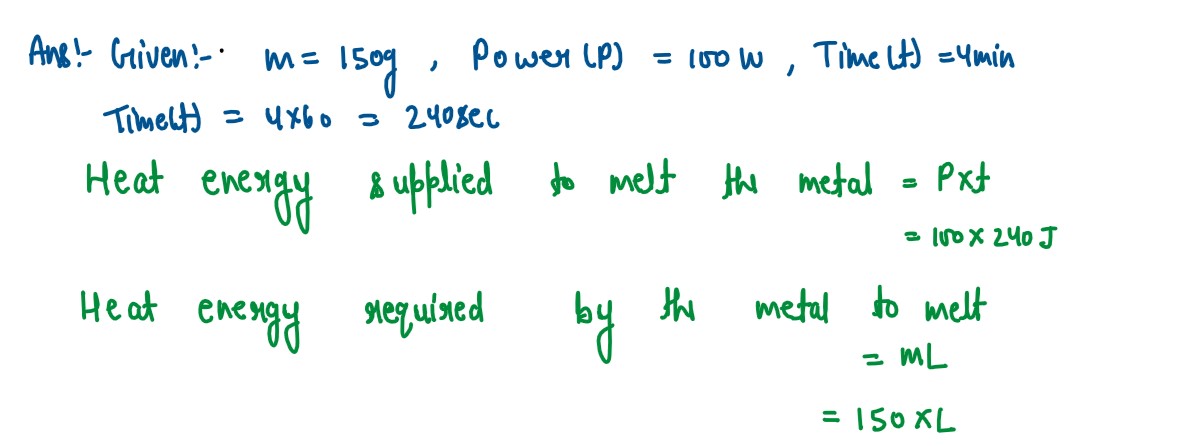
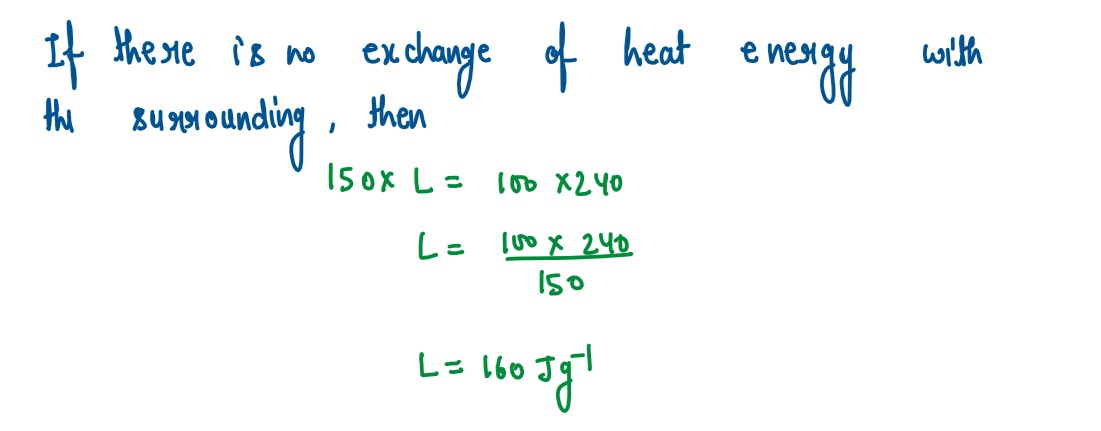
solutions
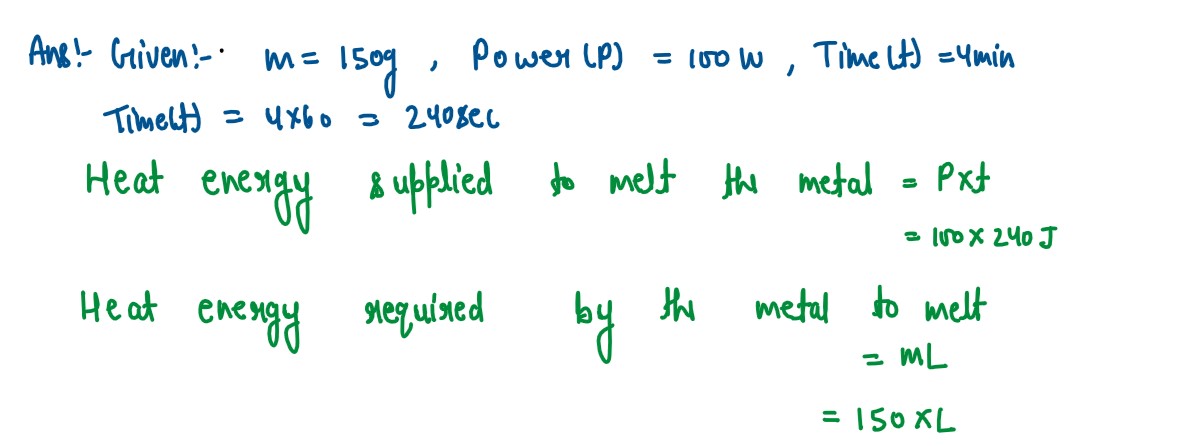
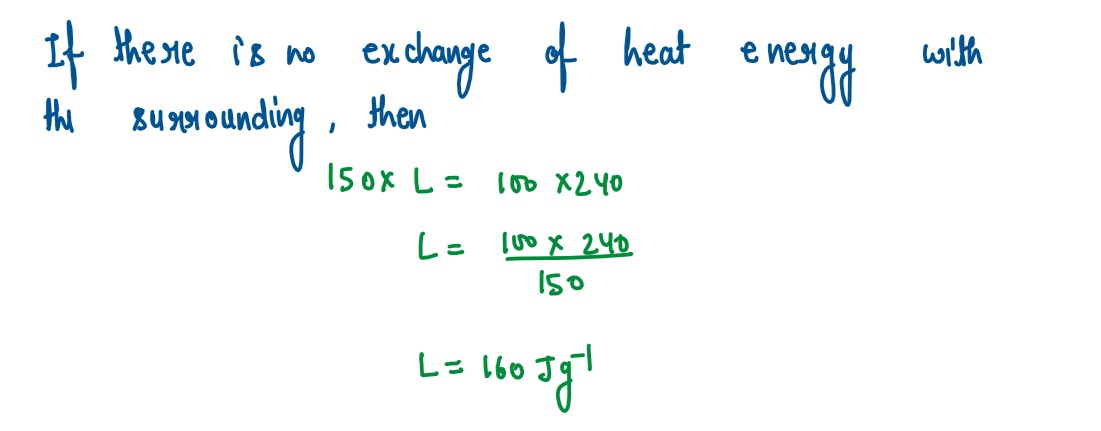
Q3
(i) Heat supplied to a solid change it into liquid. What is this change in phase called?
(ii) During the phase change does the average kinetic energy of the molecules of the
substance increase?
(iii) What is the energy absorbed during the phase change called?
solutions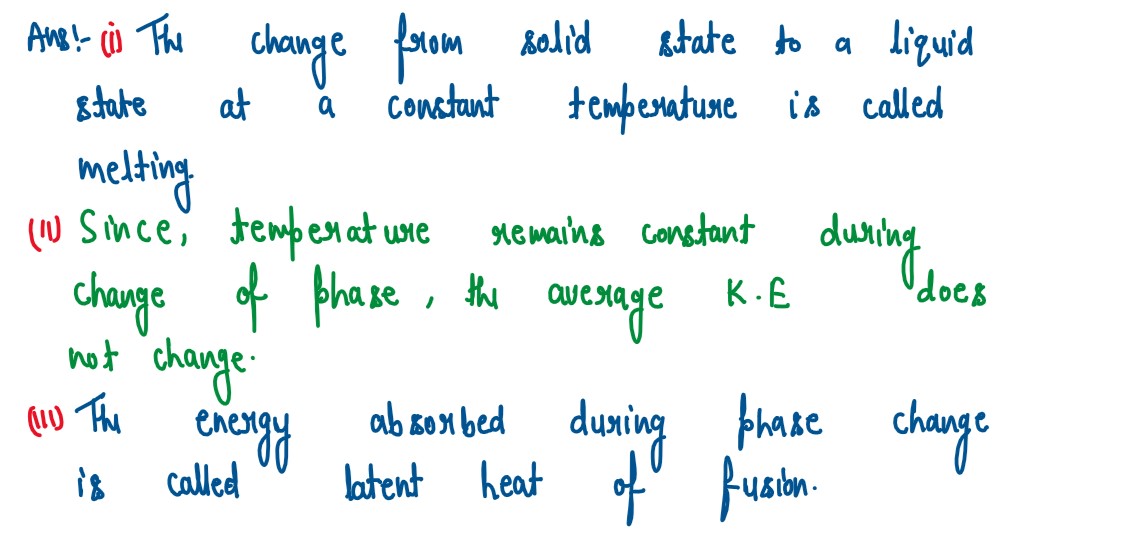
solutions

Q4
(i) State two differences between "Heat Capacity" and "Specific Heat Capacity".
(ii) Give a mathematical relation between Heat Capacity and Specific Heat Capacity.
solutions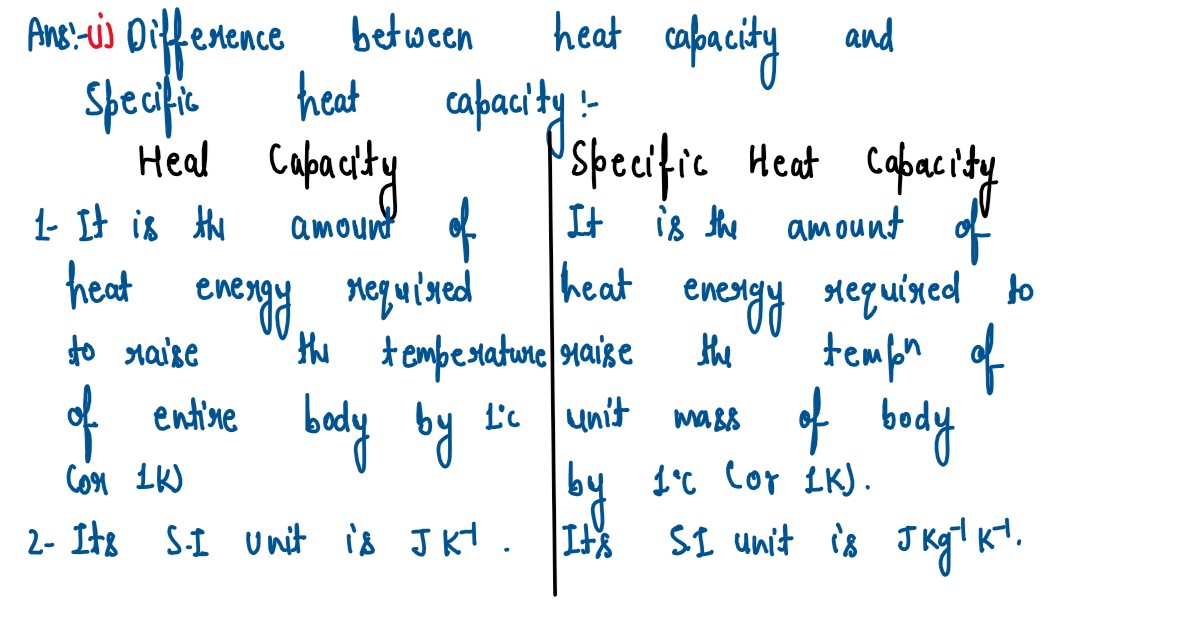
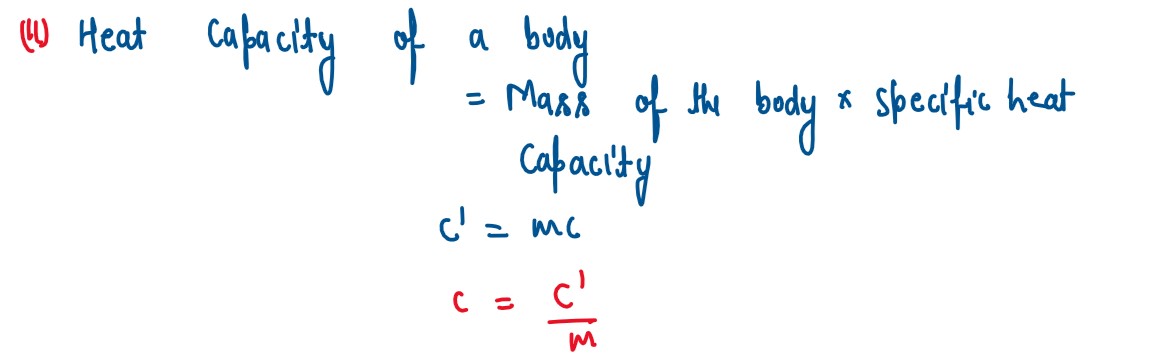
(ii) Give a mathematical relation between Heat Capacity and Specific Heat Capacity.
solutions

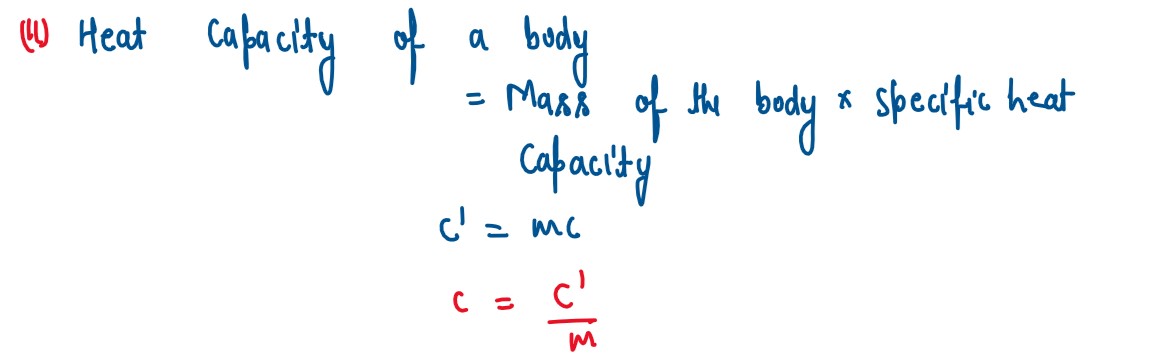
Q5
The temperature of 170g of water at 50°C is lowered to 5°C by adding a certain amount
of ice to it. Find the mass of ice added.
Given: Specific heat capacity of water = 4200 J kg-1°C-1 and Specific latent heat of ice = 336000 J kg-1
solutions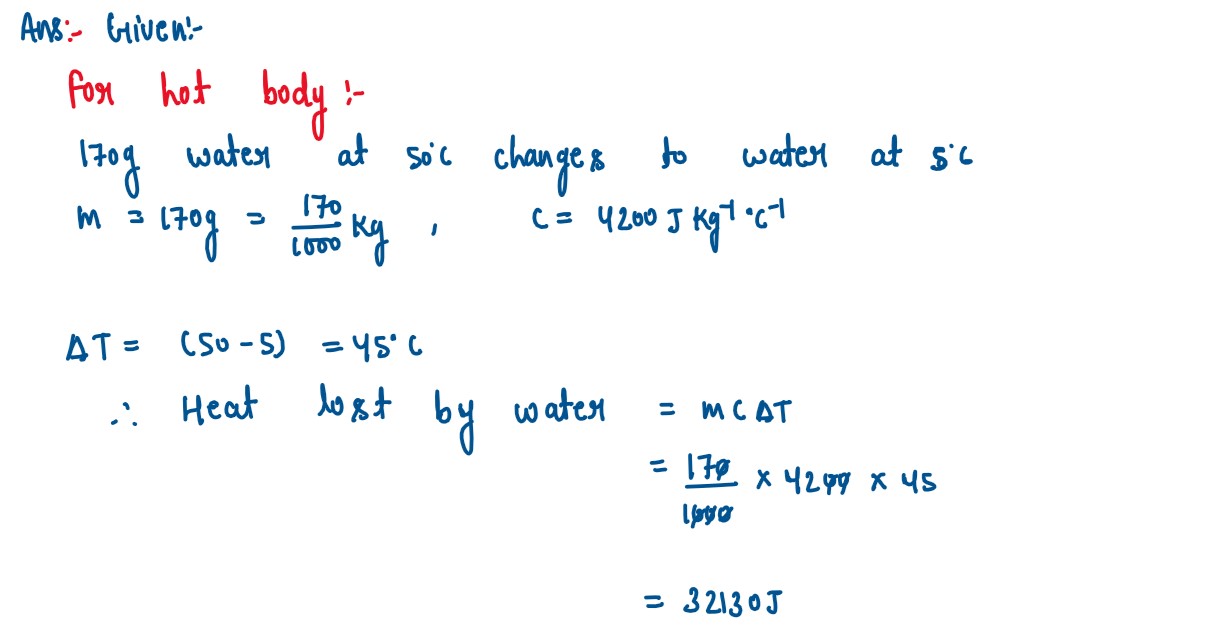
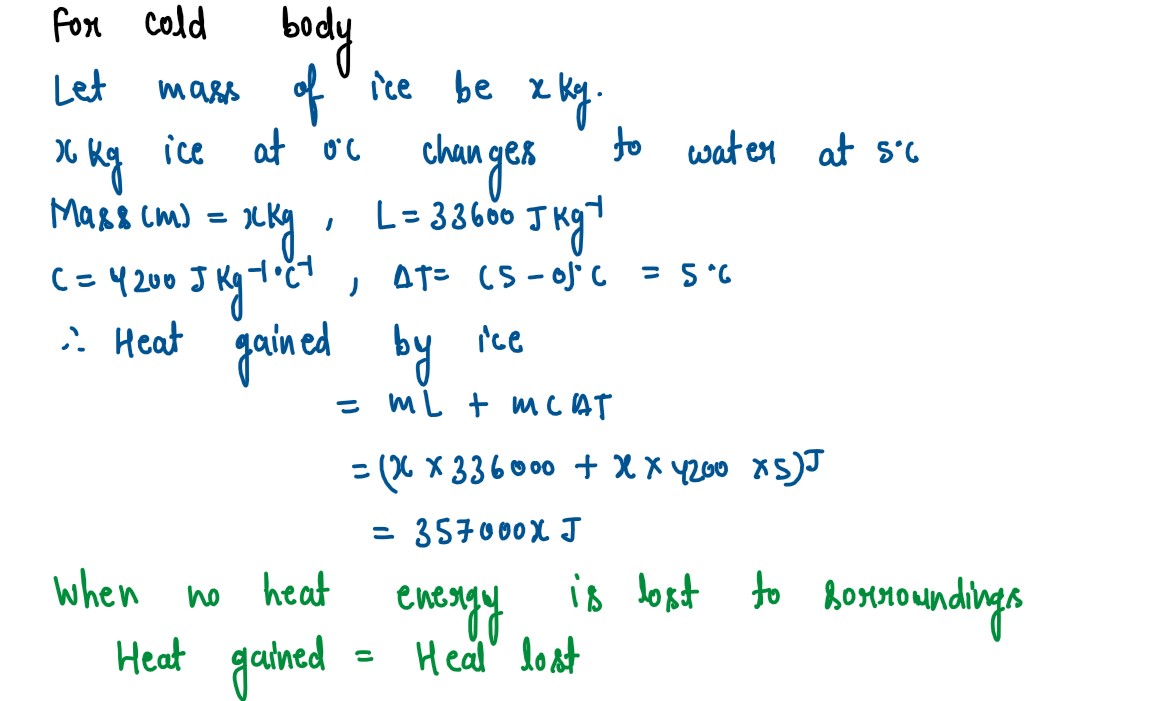
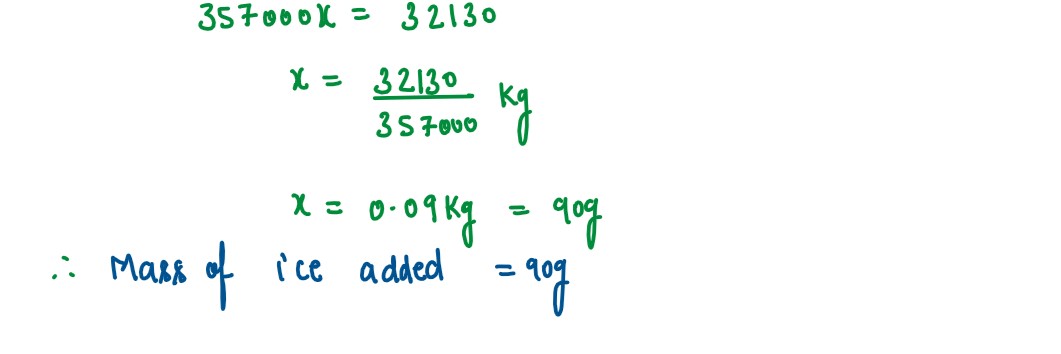
Given: Specific heat capacity of water = 4200 J kg-1°C-1 and Specific latent heat of ice = 336000 J kg-1
solutions
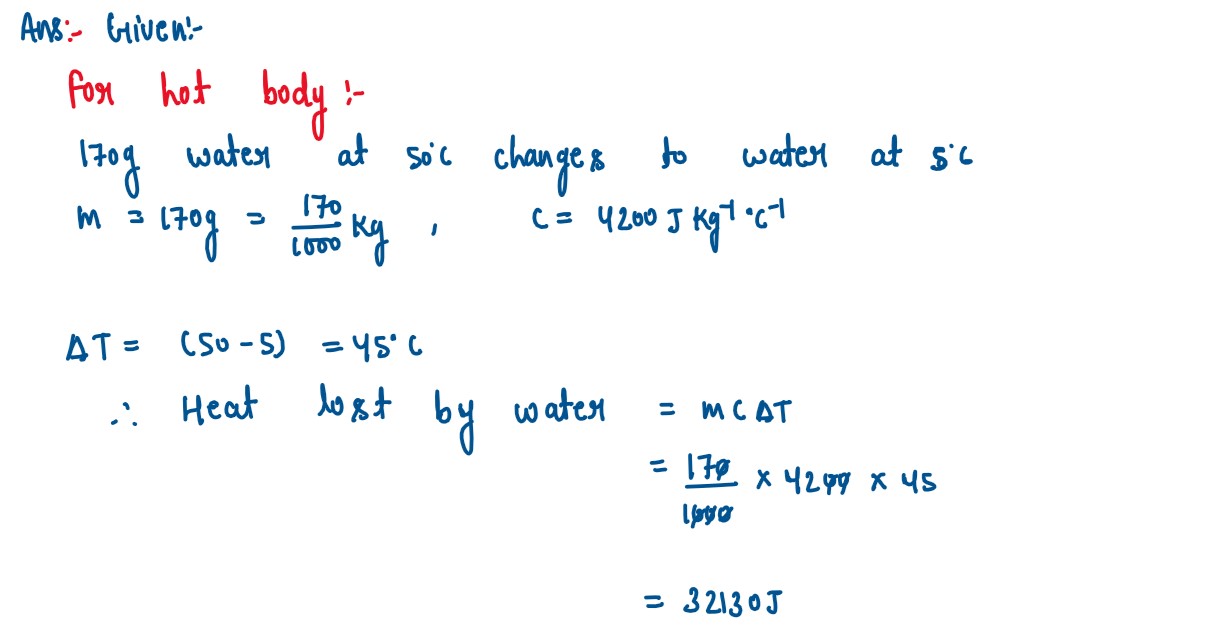
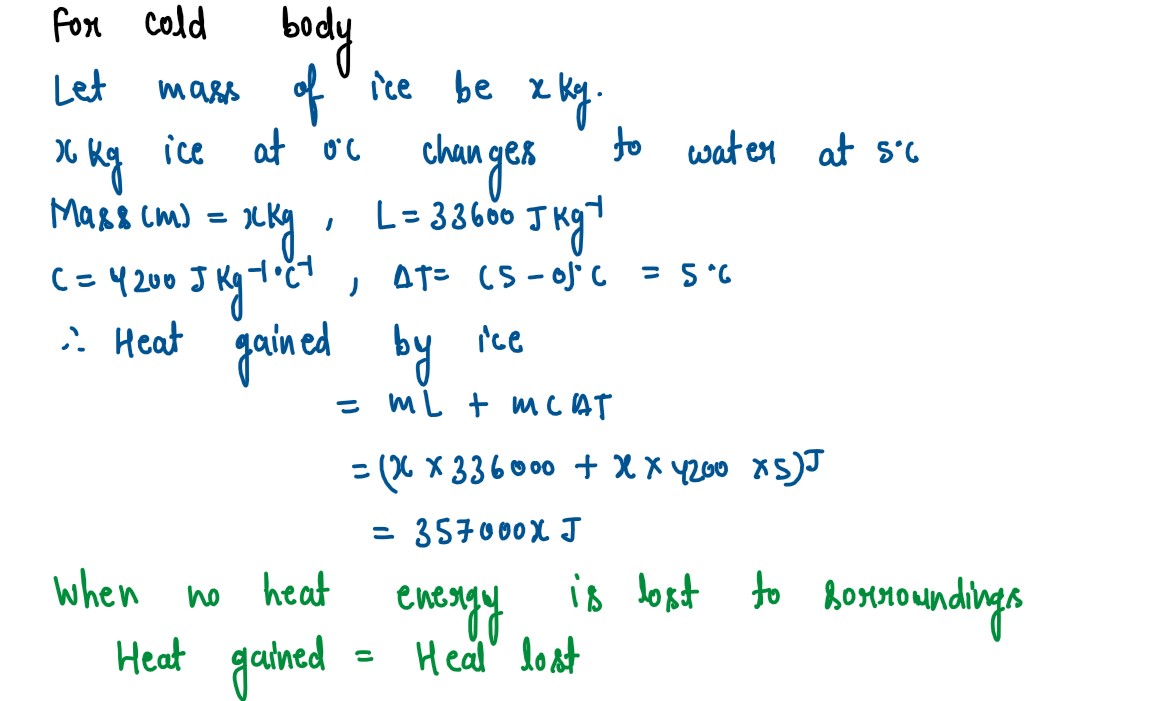
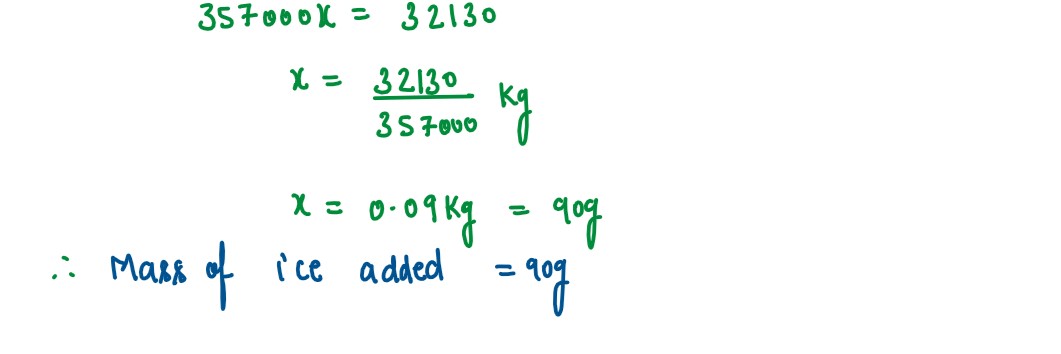
2017
Q1
Define heat capacity and its S.I unit
solutions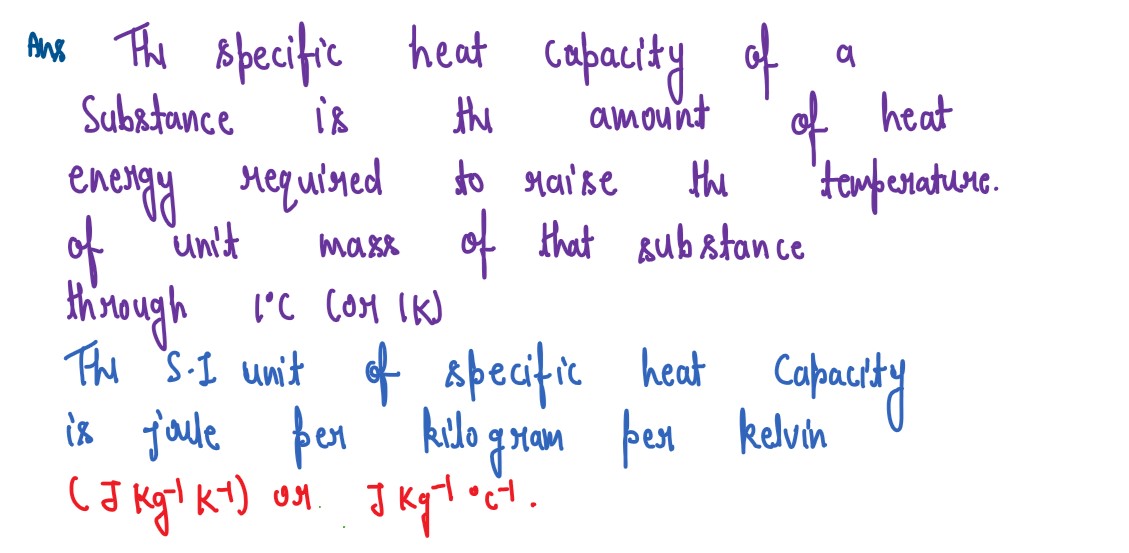
solutions

Q2
Why is the base of a cooking pan generally made thick
solutions
solutions

Q3
A solid of mass 50 g at 150°C is placed in 100 g of water at 11°C, when the final
temperature recorded is 20°C. Find the specific heat capacity of the solid.
(Specific heat capacity of water = 4.2 J/g°C)
How is the refractive index of a material related to?
solutions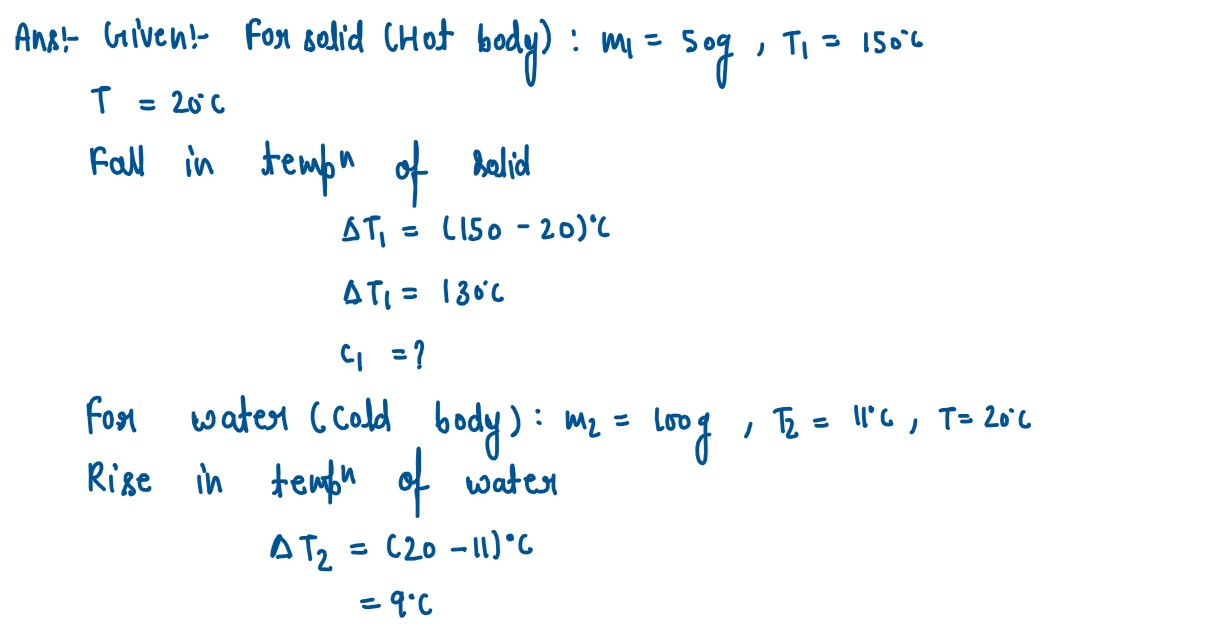
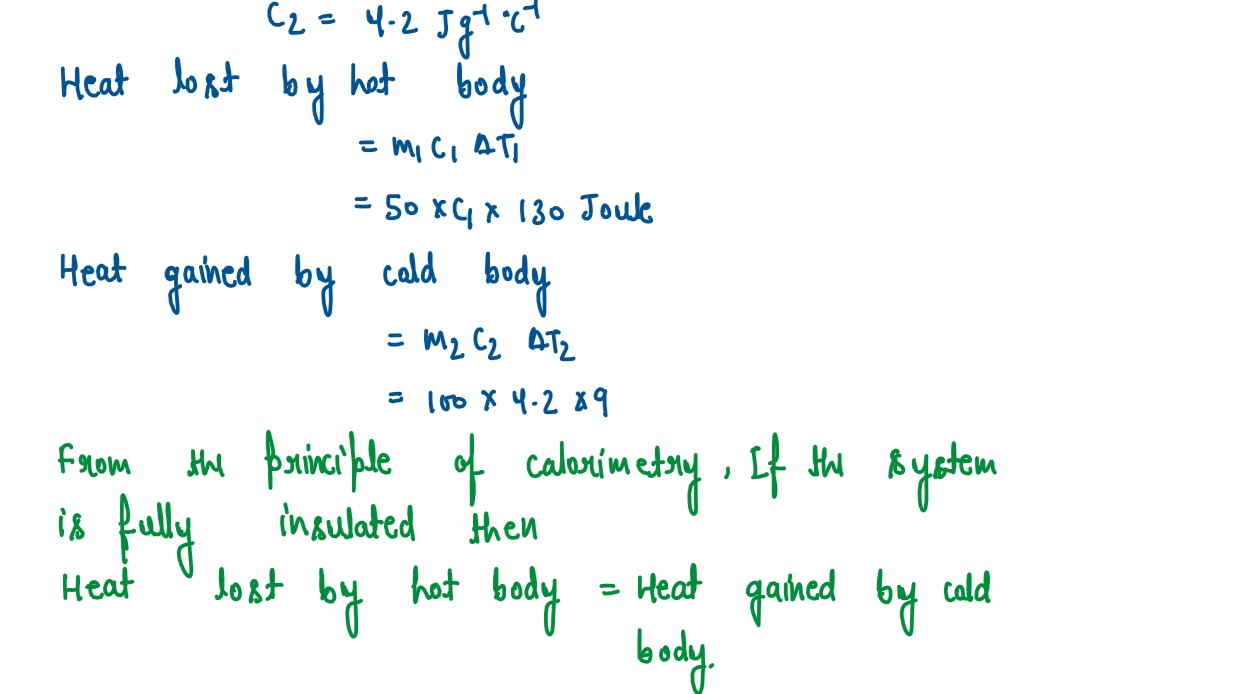
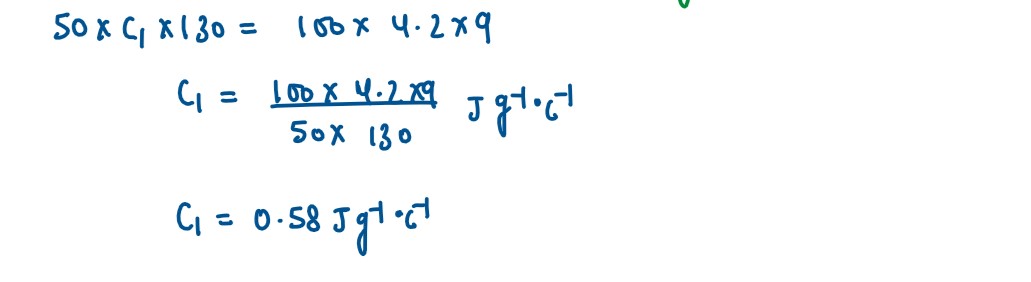
(Specific heat capacity of water = 4.2 J/g°C)
How is the refractive index of a material related to?
solutions
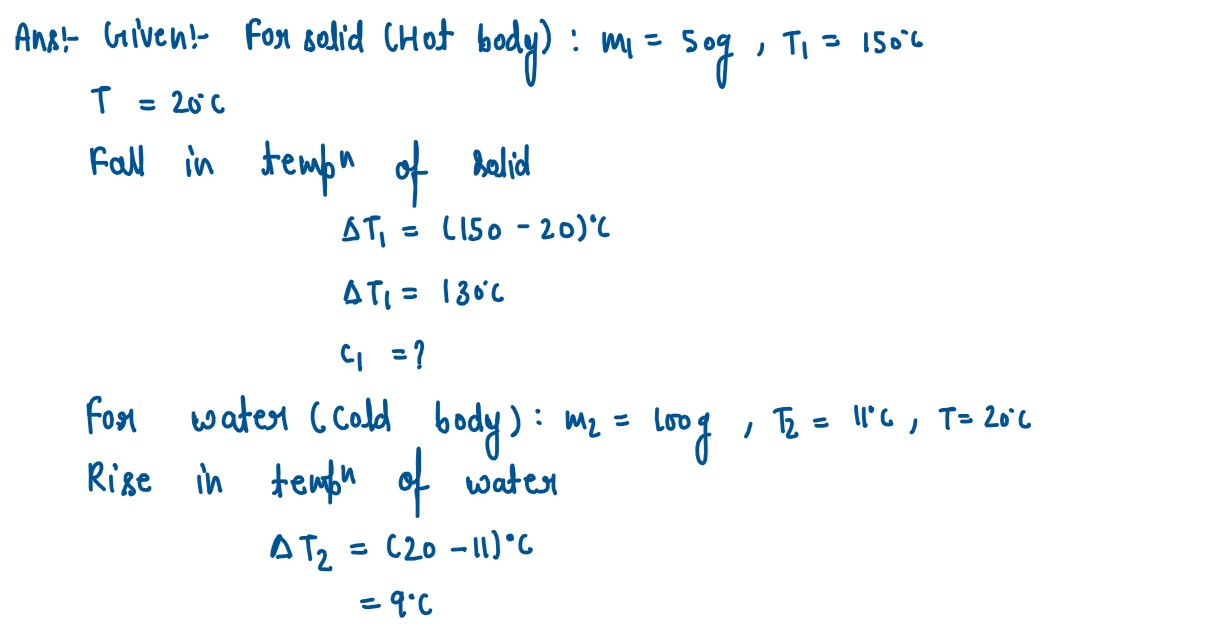
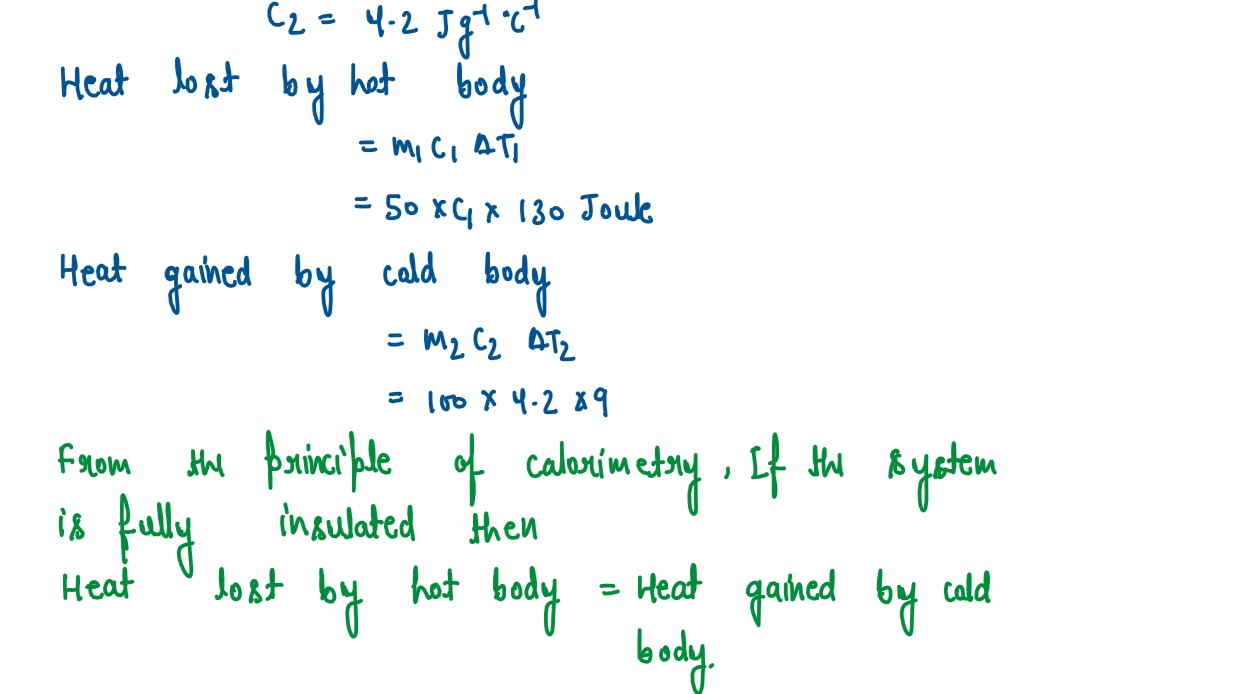
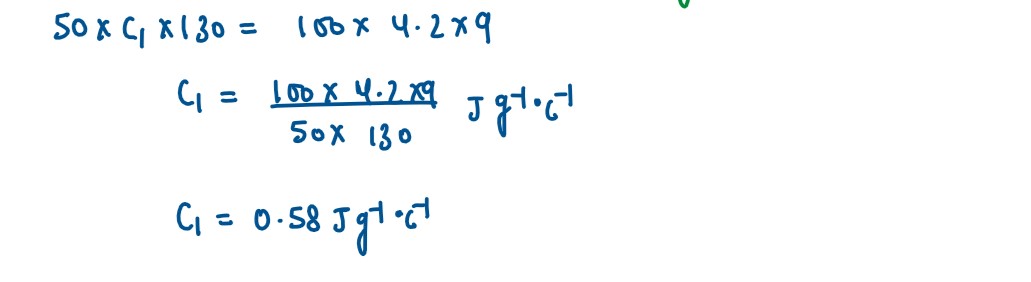
Q4
How is the transference of heat energy by radiation prevented in a calorimeter
solutions
solutions

Q5
You have a choice of three metals A, B and C, of specific heat capacities 900
Jkg-1°C-1
380 Jkg-1°C-1 and 460 Jkg-lvel respectively, to make a calorimeter. Which
material will you select? Justify answer.
solutions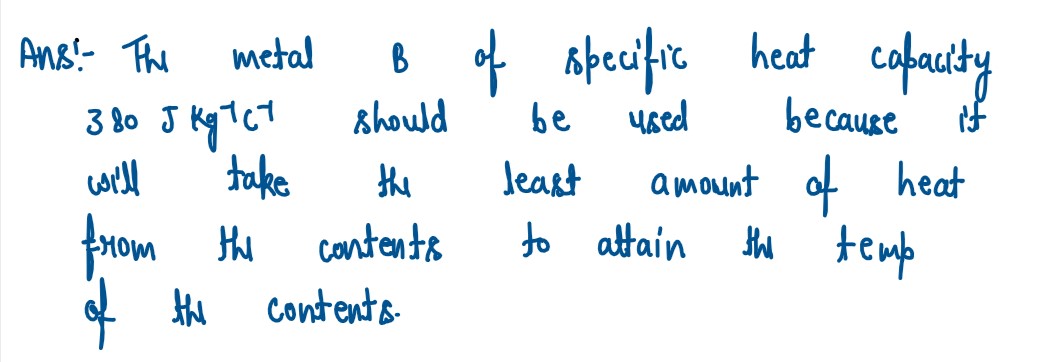
solutions

Q6
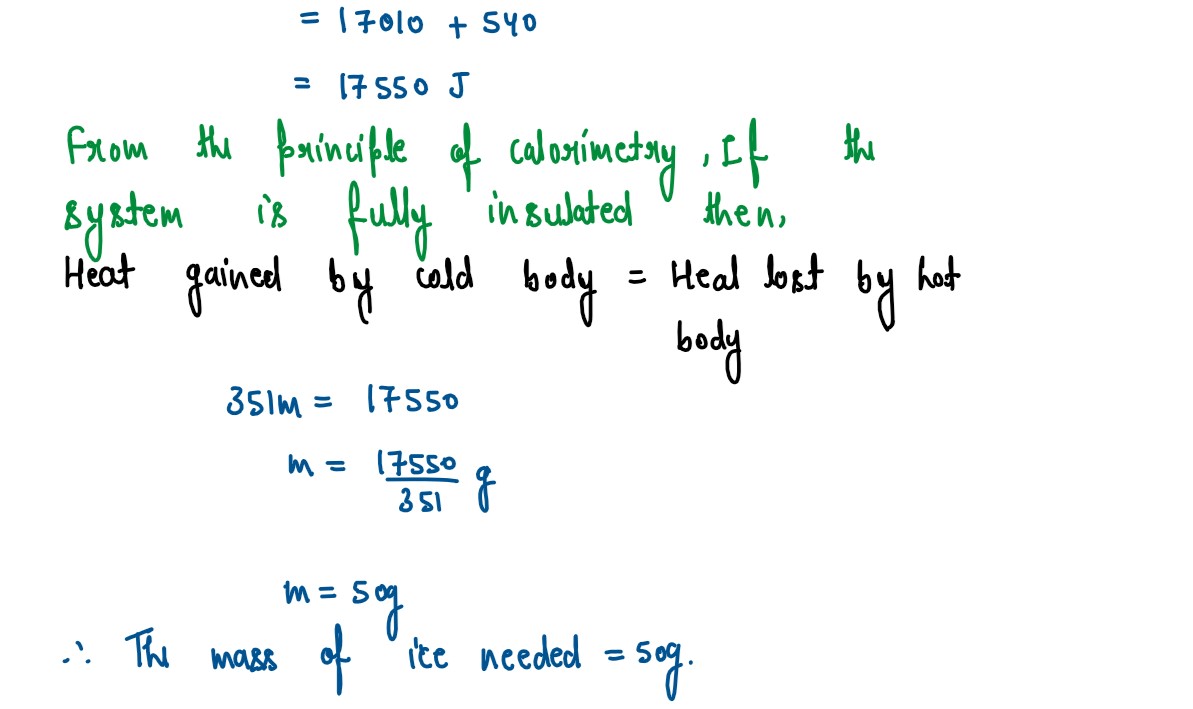
Calculate the mass of ice needed to 150g of water contained in a calorimeter of
mass 50g at 32°C such that the final temperature is 5°C.
Specific heat capacity of calorimeter = 0.4 J/g°C
Specific heat capacity of water=4.2 J/g°C
Latent heat capacity of ice=330 J/g
solutions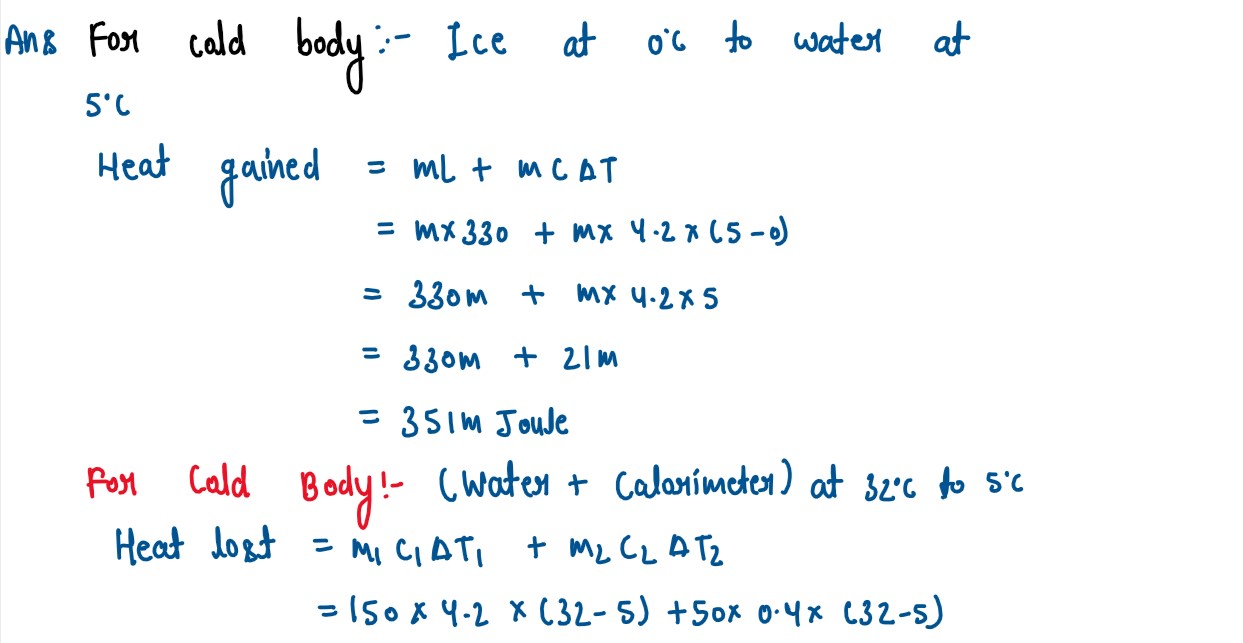
Specific heat capacity of water=4.2 J/g°C
Latent heat capacity of ice=330 J/g
solutions

2016
Q1
Calculate the mass ofice required to lower the temperature of 300g ofwater at 40°C to
water at O°C.
(Specific latent heat of ice=336 J/g, Specific heat capacity of wateA.2 J/g°C)
solutions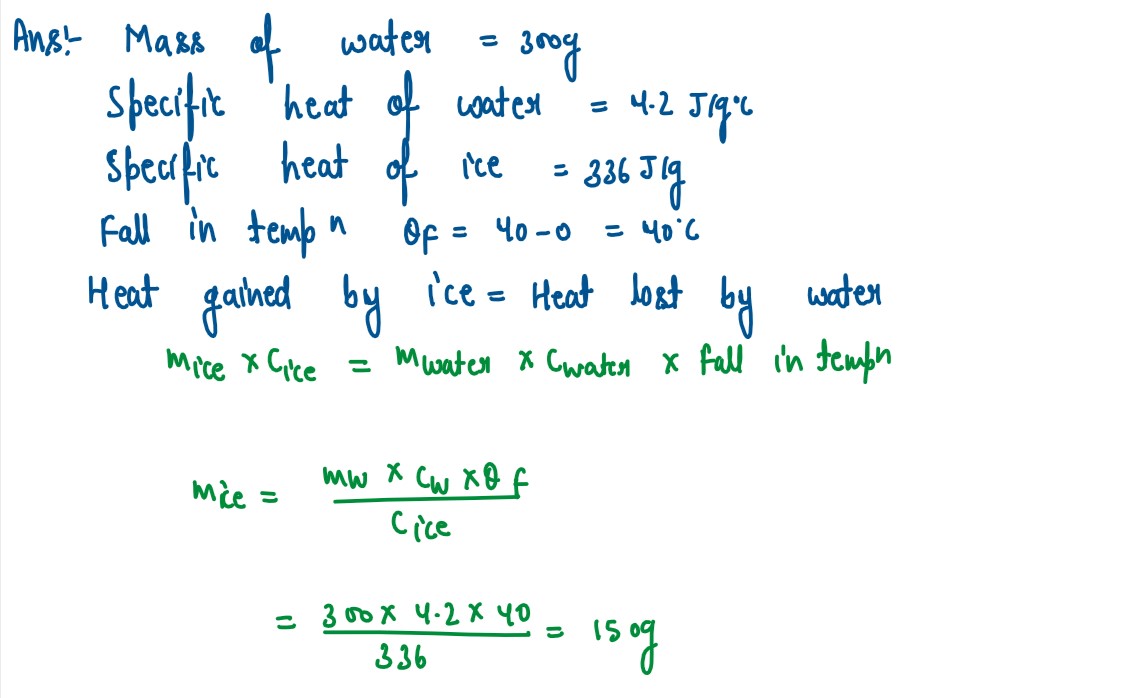

(Specific latent heat of ice=336 J/g, Specific heat capacity of wateA.2 J/g°C)
solutions
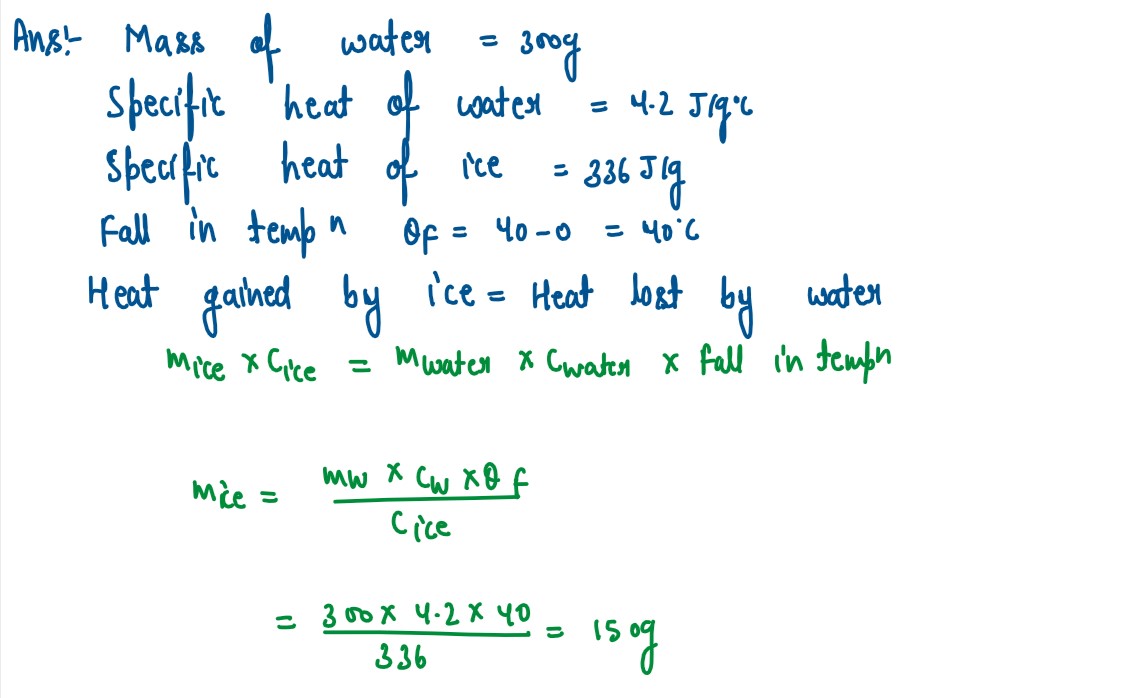

Q2
What do you understand by the follow in statements:
(i) The heat capacity of the body is 60 JK-1.
(ii) The specific heat capacity of lead is 130 JKg-1K-1
solutions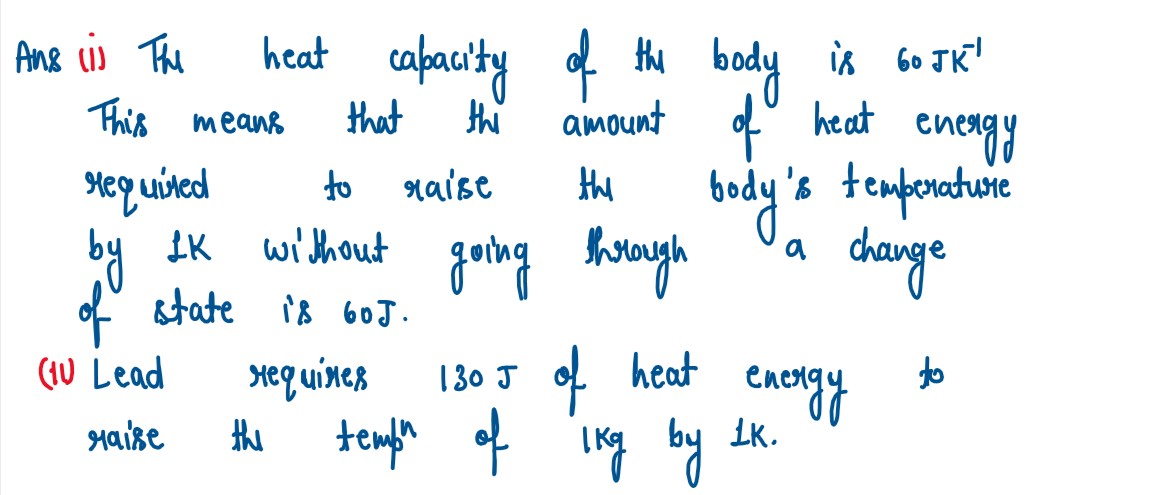
(i) The heat capacity of the body is 60 JK-1.
(ii) The specific heat capacity of lead is 130 JKg-1K-1
solutions
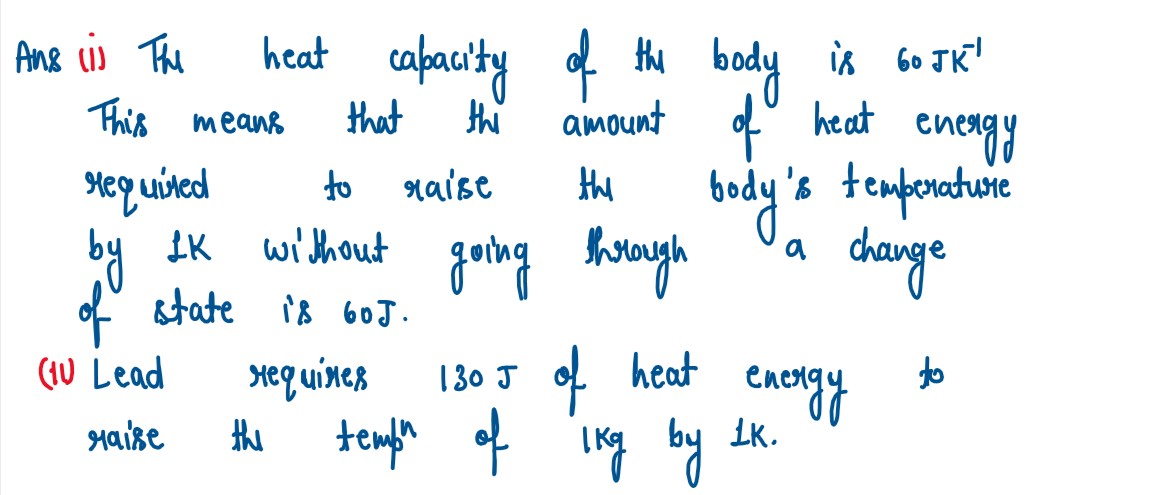
Q3
State two factors upon which the heat absorbed by a body depends
solutions
solutions

Q4
(i) What is the principle of method of mixtures?
(ii) What is the Other name given to it?
(iii) Name the law on which the principle is based.
solutions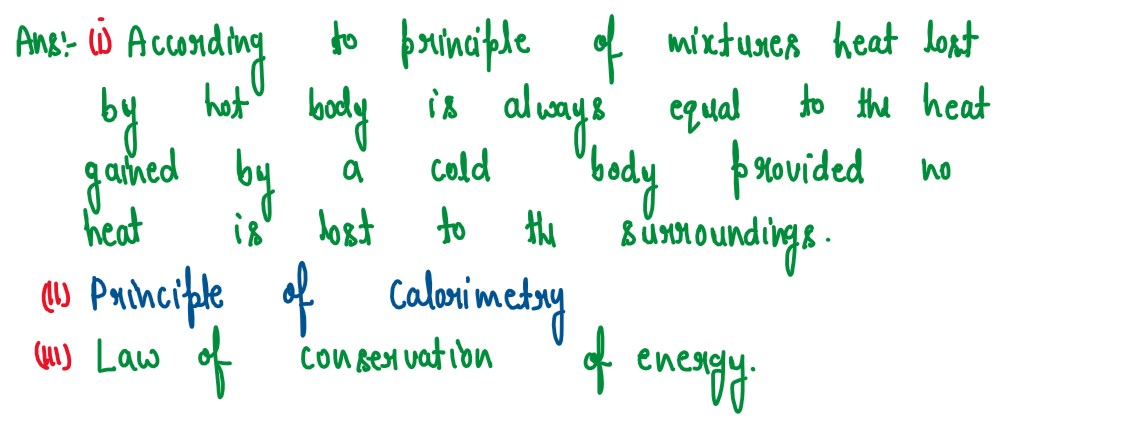
(ii) What is the Other name given to it?
(iii) Name the law on which the principle is based.
solutions
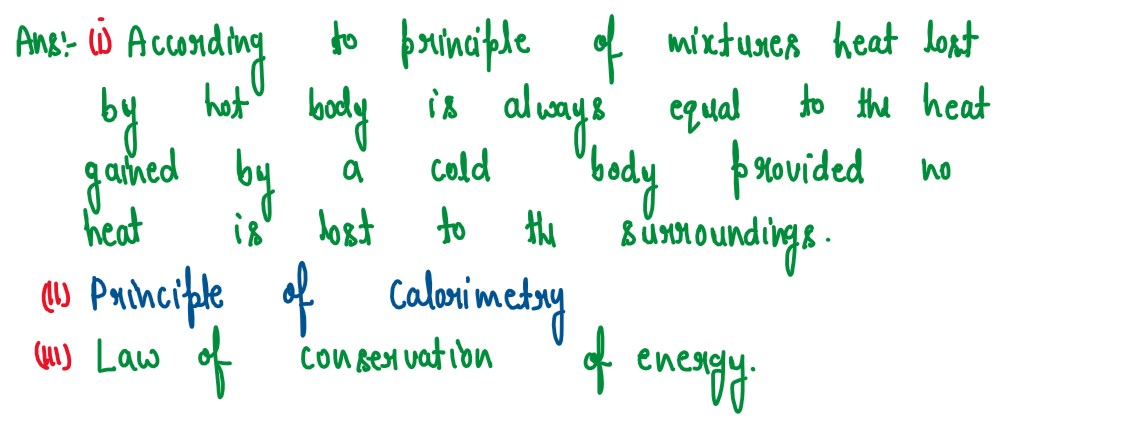
Q5
Some ice is heated al a constant rate, and its temperature is recorded after every few
seconds, till steam is formed at 100°C. Draw a temperature time graph to represent the
change. Label the two phase changes in your graph.
solutions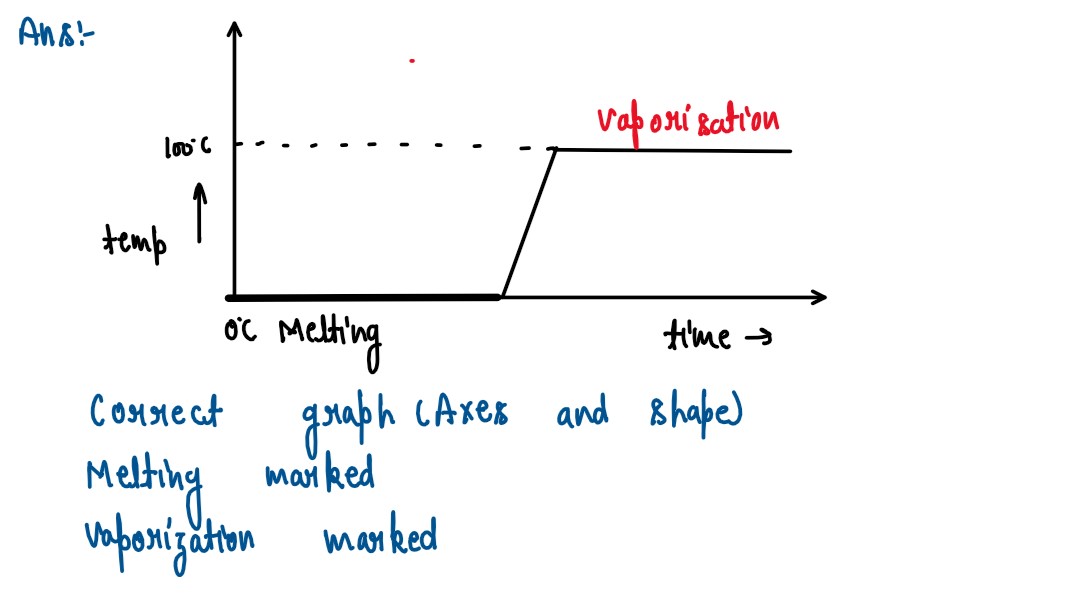
solutions

Q6
A copper vessel of mass 100 g contains 150 g ofwater at 500C. How much ice is needed
to cool it to 5°C?
Given : Specific heat capacity of copper= 0.4 Jg-1 °C-1
Specific heat capacity of water =4.2 Jg-1 °C-1
Specific latent heat of fusion of ice = 336Jg-1
solutions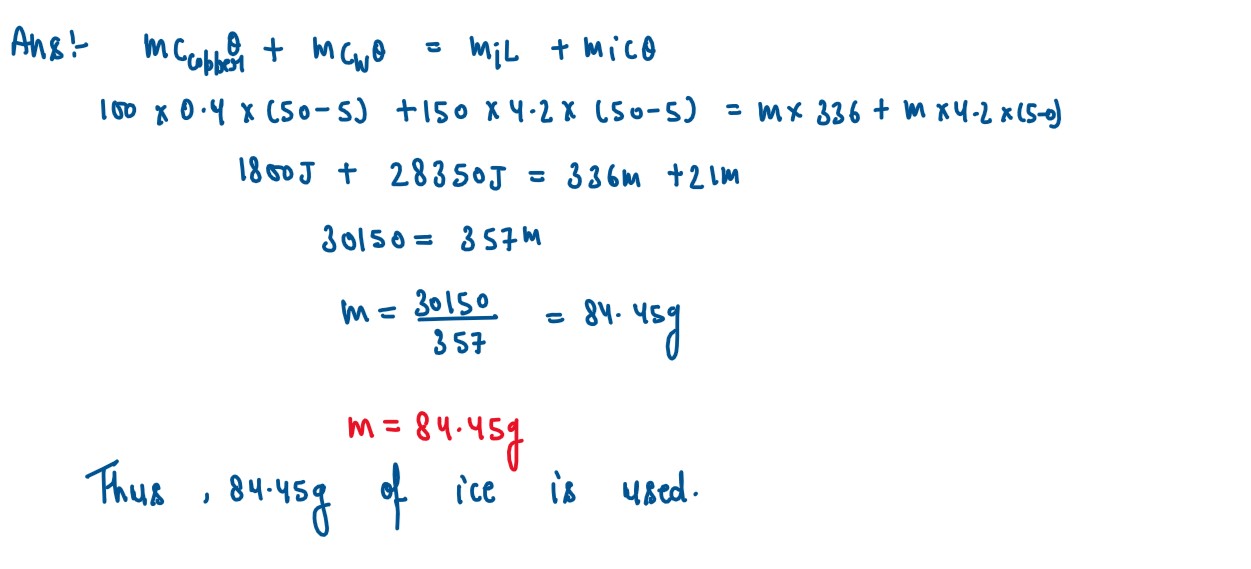
Given : Specific heat capacity of copper= 0.4 Jg-1 °C-1
Specific heat capacity of water =4.2 Jg-1 °C-1
Specific latent heat of fusion of ice = 336Jg-1
solutions
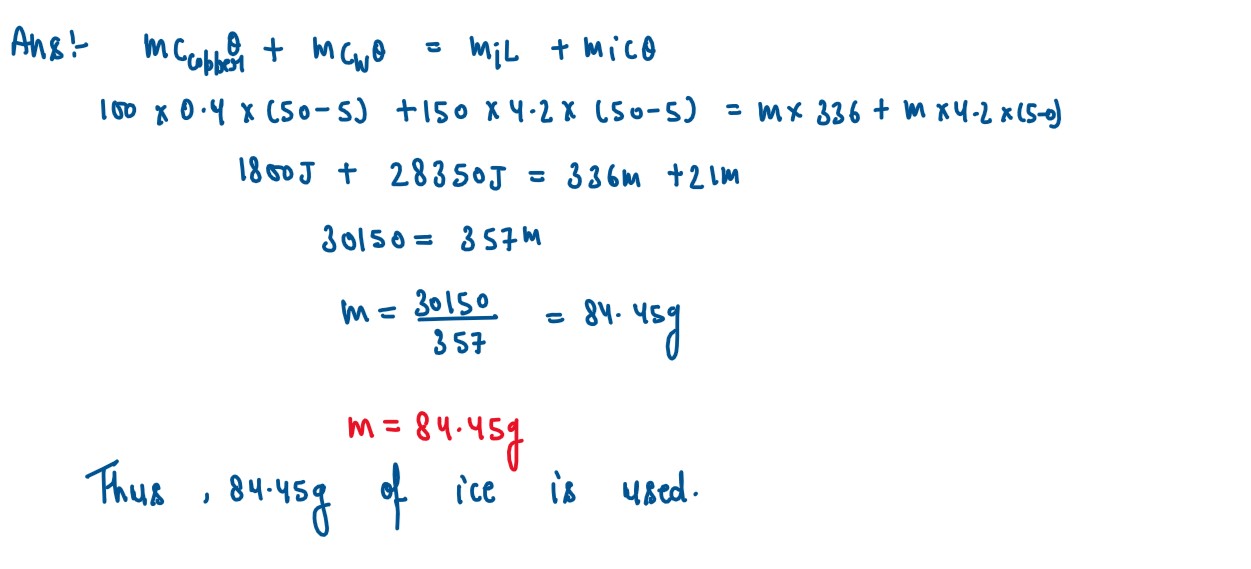

Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment