Chemical Reaction & Equations Chapter Board Questions Class 10 CBSE
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Chemical Reaction & Equations. These important notes,board questions and predicted questions are based on CBSE. board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
Calcium Oxide reacts vigorously with water to produce slaked lime
CaOs + H2O l → Ca(OH)aq
This reaction can be classified as :
(A) Combination Reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Which of the following is the correct option?
solutions
CaOs + H2O l → Ca(OH)aq
This reaction can be classified as :
(A) Combination Reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Which of the following is the correct option?
solutions

Q2
When hydrogen sulphide gas is passed through a blue solution of copper sulphate a black precipitate
of copper sulphide is obtained and the sulfuric chemical sow formed remains in the solution.The
reaction is an example of a
(A) Combination reaction
(B) Displacement reaction
(C) Decomposition reaction
(D) Double displacement reaction
solutions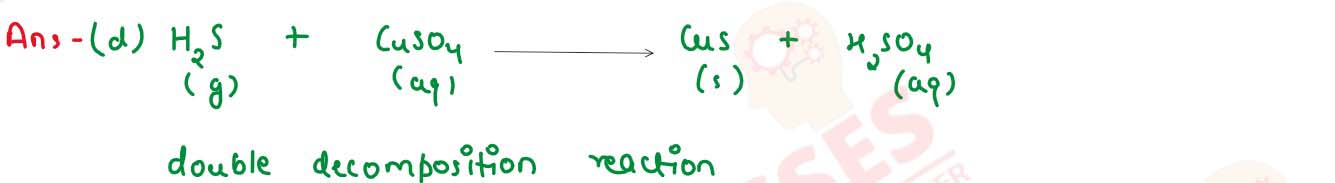
(A) Combination reaction
(B) Displacement reaction
(C) Decomposition reaction
(D) Double displacement reaction
solutions

Q3
In a double displacement s such reaction the reaction between sodium sulphate solution and barium
chloride solution:
(A) Exchange of atoms take place
(B) Exchange of ions take place
(C) A precipitate is produced
(D) An insoluble salt is produced
The correct option is
(a) (B) and (D)
(b) (A) and (C)
(c) Only (B)
(d) (B), (C) and (D)
solutions
(A) Exchange of atoms take place
(B) Exchange of ions take place
(C) A precipitate is produced
(D) An insoluble salt is produced
The correct option is
(a) (B) and (D)
(b) (A) and (C)
(c) Only (B)
(d) (B), (C) and (D)
solutions

Q4
The position of certain elements in the modern periodic table is shown below

Using the above table answer the following questions giving reasons in each case :
(i) Which element will form only covalent compounds ?
(ii) Which element is a non-metal with valency 2 ?
(iii) Which element is a metal with valency 2 ?
(iv) Out of H, C and F which has the largest atomic size ?
(v) To which family does H, C and F belong ?
solutions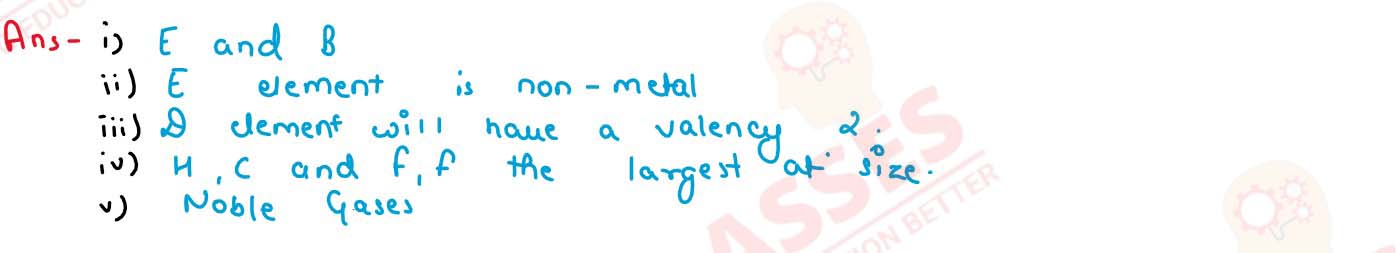

Using the above table answer the following questions giving reasons in each case :
(i) Which element will form only covalent compounds ?
(ii) Which element is a non-metal with valency 2 ?
(iii) Which element is a metal with valency 2 ?
(iv) Out of H, C and F which has the largest atomic size ?
(v) To which family does H, C and F belong ?
solutions
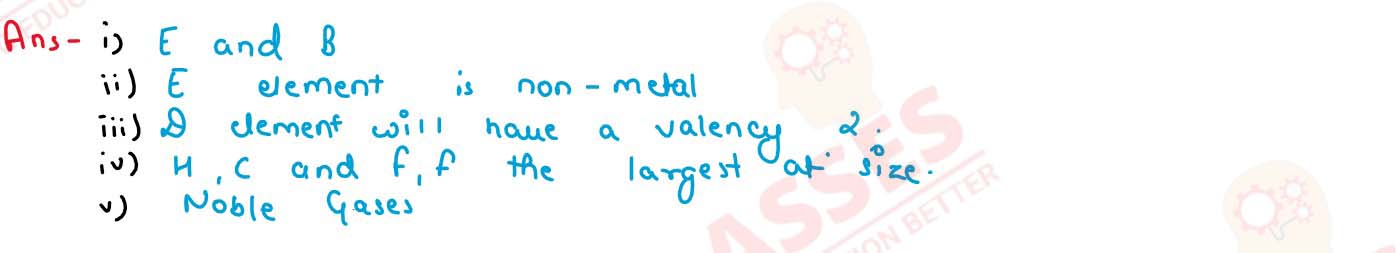
2019
Q5
13.2 g of silver chloride is taken in a China dish and the China dish is placed in sunlight for some
time.What will be your observation in this case? Write the chemical reaction involved in the form of
a balanced chemical equation identify the type of chemical reaction.
OR
The type of reactions taking place in each of the following cases and write the balanced chemical equation for the reactions
(a) Zinc reacts with silver nitrate to produce zinc nitrate and silver
(b) Potassium iodide react with lead nitrate to produce potassium nitrate and lead iodide
solutions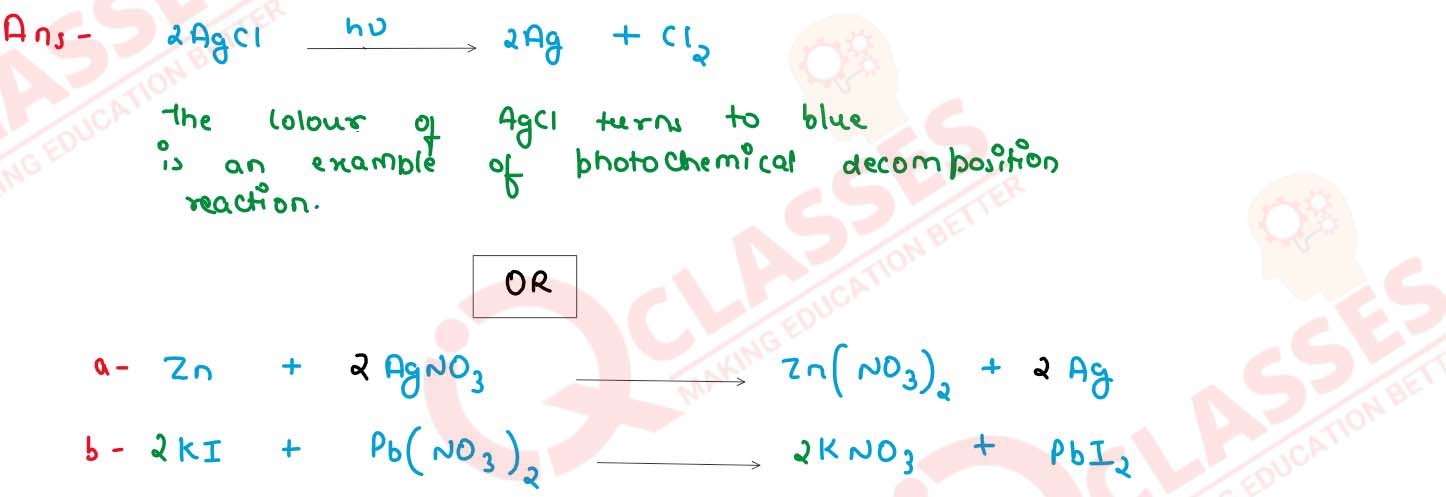
The type of reactions taking place in each of the following cases and write the balanced chemical equation for the reactions
(a) Zinc reacts with silver nitrate to produce zinc nitrate and silver
(b) Potassium iodide react with lead nitrate to produce potassium nitrate and lead iodide
solutions

Q6
Explain the following
(a) Sodium chloride is an ionic compound which does not conduct electricity in solid state whereas it does conduct electricity molten state as well as in aqueous solution
(b) Reactivity of aluminium decreases if it is dipped in nitric chemical
(c) Metals like calcium and magnesium are never found in their free State in nature
solutions
(a) Sodium chloride is an ionic compound which does not conduct electricity in solid state whereas it does conduct electricity molten state as well as in aqueous solution
(b) Reactivity of aluminium decreases if it is dipped in nitric chemical
(c) Metals like calcium and magnesium are never found in their free State in nature
solutions

2018
Q7
Decomposition reaction require energy either in the form of heat or light or electricity for
breaking down the reactants. Write one equation each for decomposition reaction where energy is
supplied in the form of heat, light and electricity.
solutions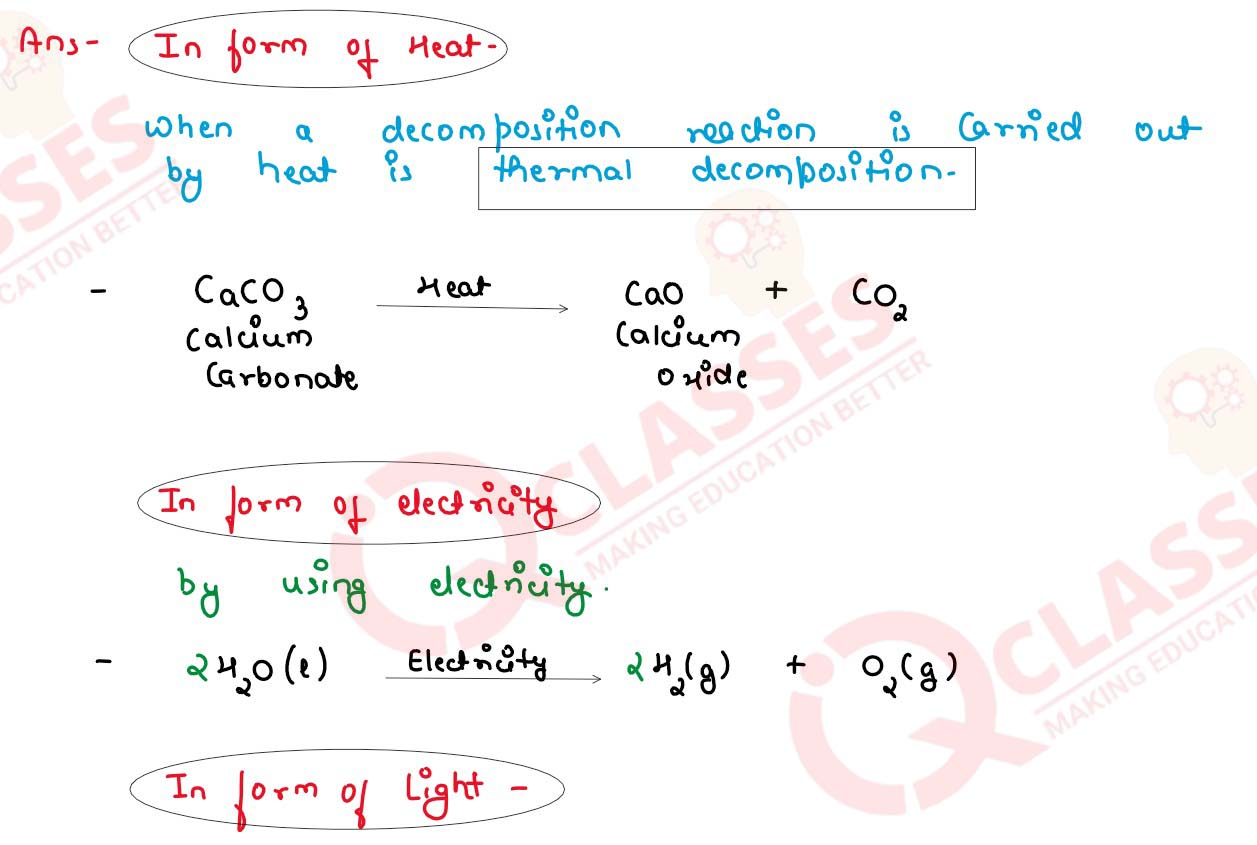
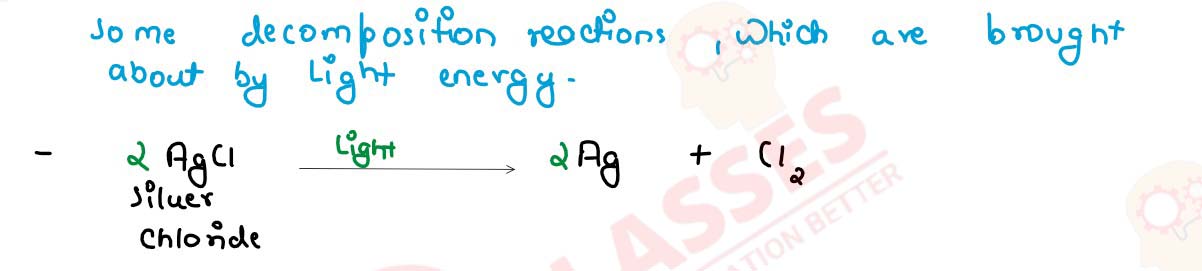
solutions



Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment