Class 10 Chemistry ICSE Ammonia Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Ammonia. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
State one ,relevant observation for each of the following reactions:
Addition of excess ammonium hydroxide into copper sulphate solution.
solutions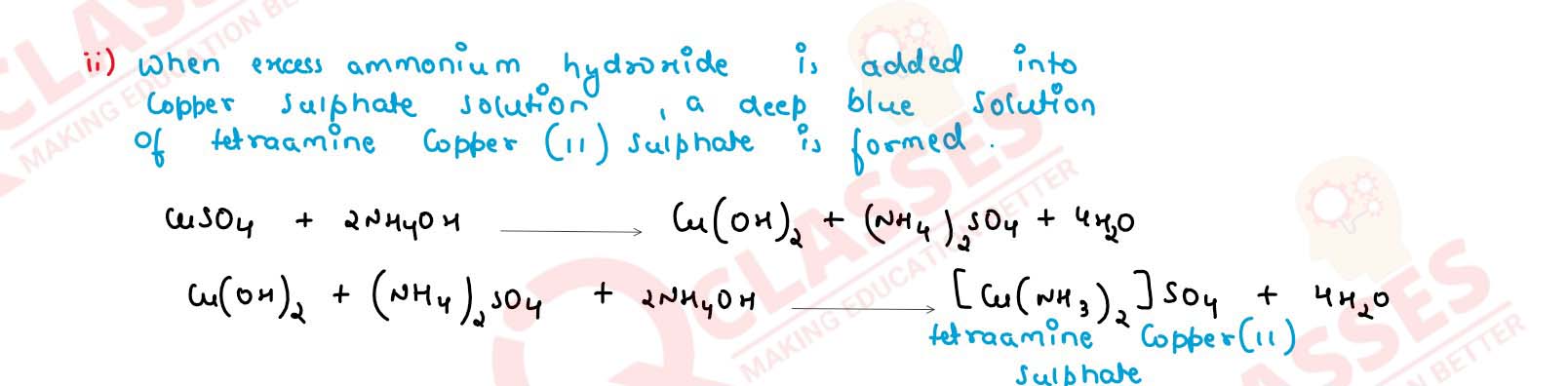
Addition of excess ammonium hydroxide into copper sulphate solution.
solutions

Q2
Write a balanced chemical equation for each of the following:
(i) Reaction of excess ammonia with chlorine.
(ii) Reaction of lead nitrate solution with ammonium hydroxide.
solutions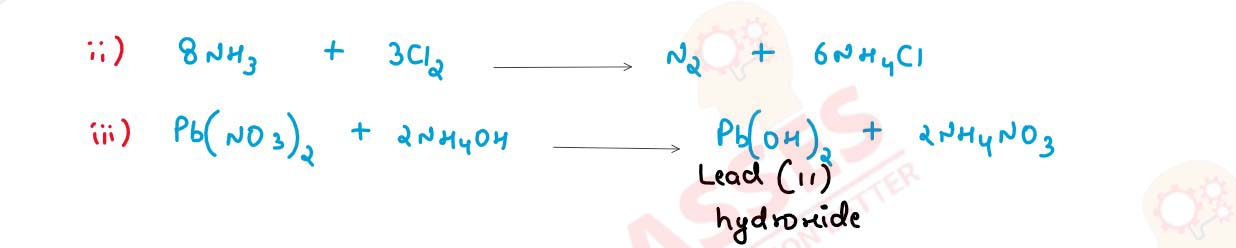
(i) Reaction of excess ammonia with chlorine.
(ii) Reaction of lead nitrate solution with ammonium hydroxide.
solutions

2019
Q3
Name the gas evolved in the following cases:
Ammonia reacts with heated copper oxide
solutions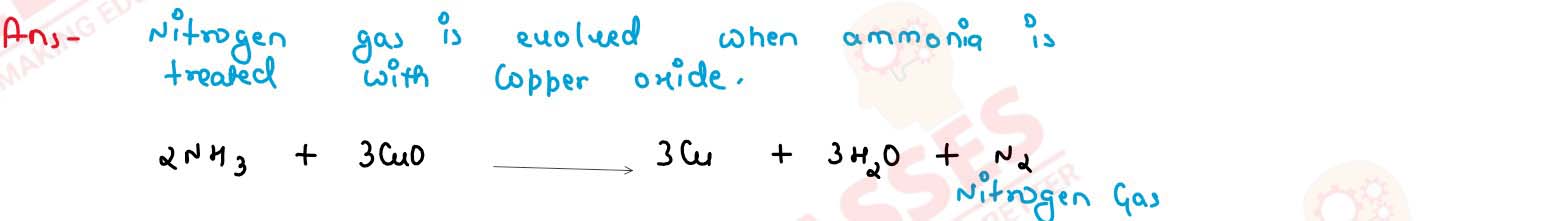
Ammonia reacts with heated copper oxide
solutions
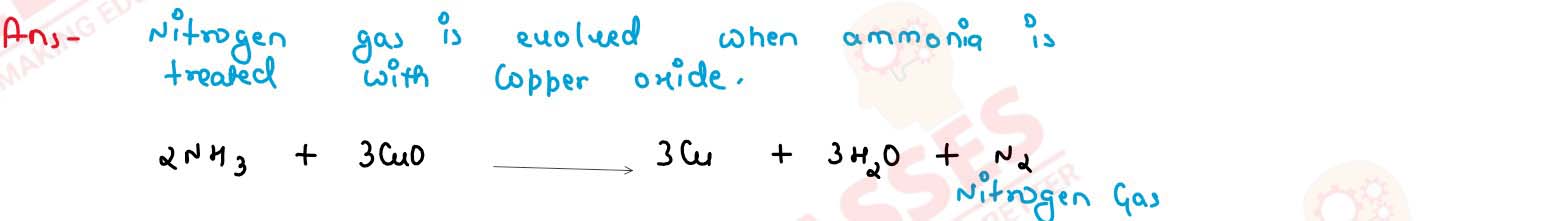
Q4
(a) Study the flow chart given and give balanced equation to represent the reaction A,B and
C
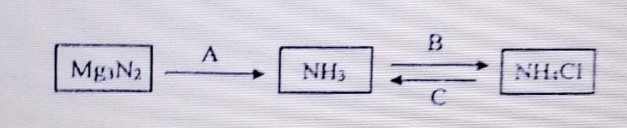
(b) Copy and complete the following table which refers to the industrial method for the preparation of ammonia and sulphuric acid:
solutions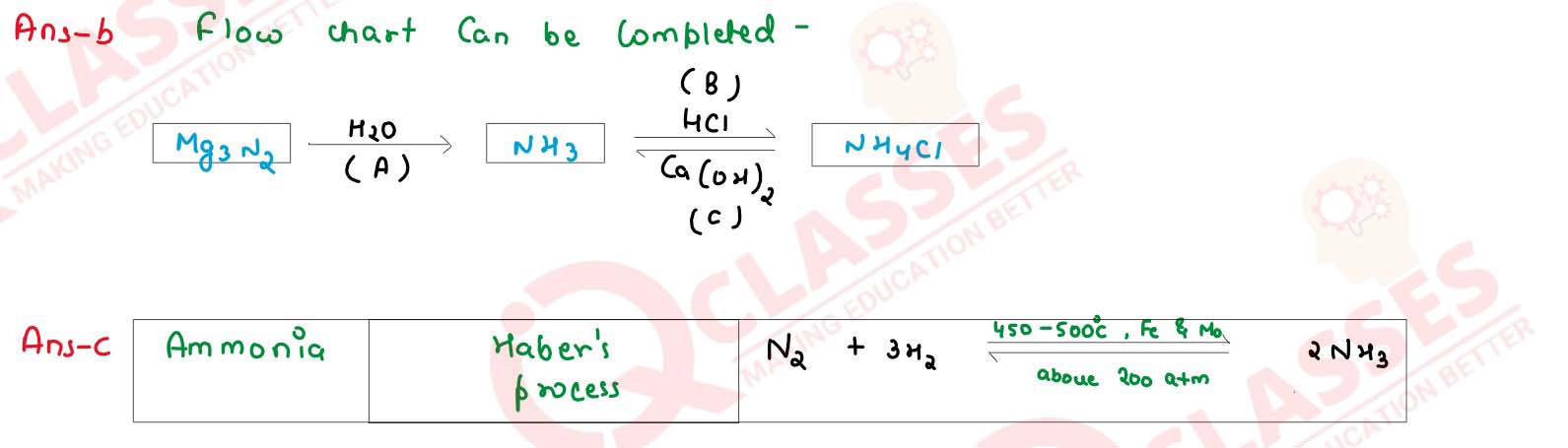

(b) Copy and complete the following table which refers to the industrial method for the preparation of ammonia and sulphuric acid:
| Name of the compound | Name of the process | Catalytic equation (with the catalyst) |
|---|---|---|
| Ammonia | (i)................. | (ii)................ |
solutions

Q5
Ammonia reacts with excess chlorine to form.....................(nitrogen / nitrogen trichloride /
ammonium chloride)
solutions
solutions

2018
Q6
Write the balanced chemical equation to prepare ammonia gas in the laboratory by using an
alkali.
(i) State why concentrated sulphuric acid is not used for drying ammonia gas.
(ii) Why is ammonia gas not collected over water?
solutions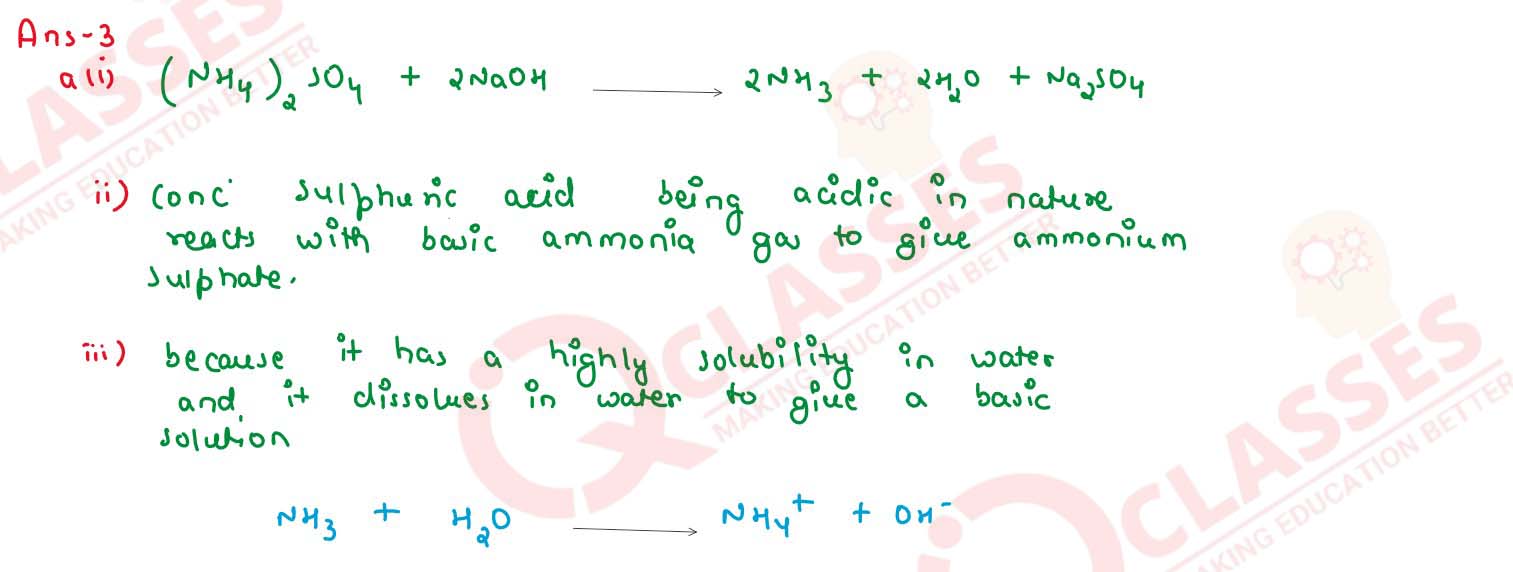
(i) State why concentrated sulphuric acid is not used for drying ammonia gas.
(ii) Why is ammonia gas not collected over water?
solutions
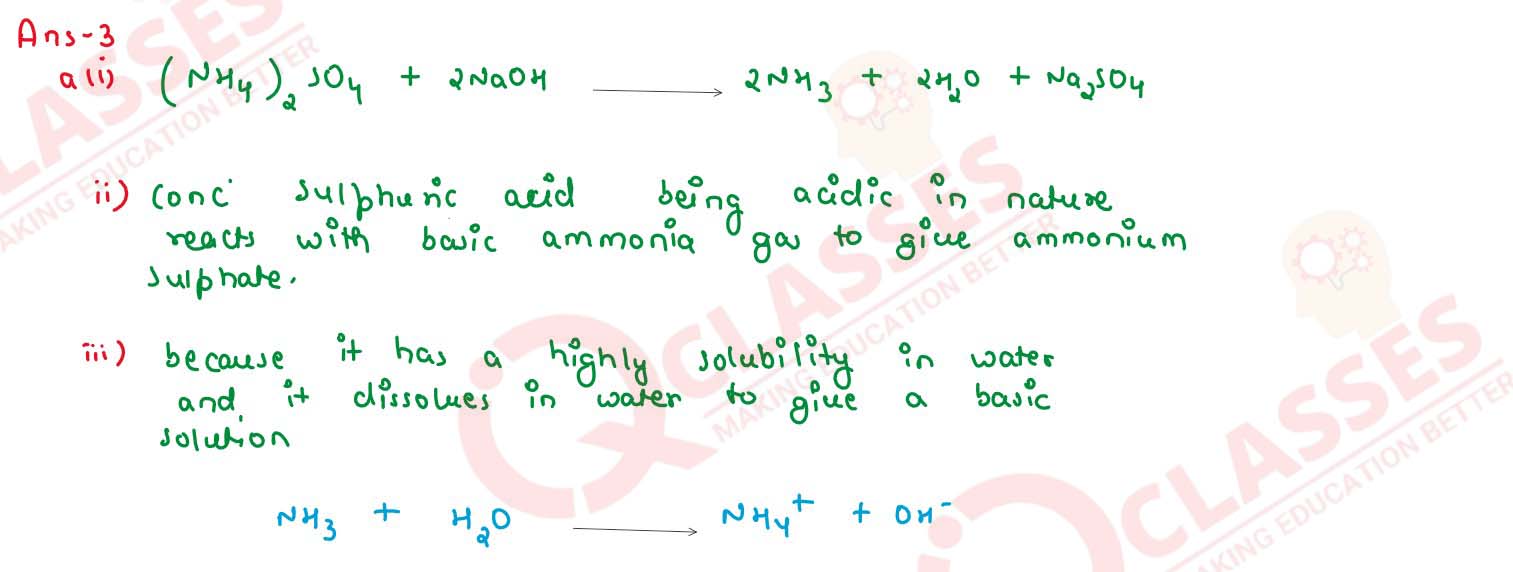
2017
Q7
Write balance equation for each of the following:
(i) reaction of ammonia with heated copper oxide
(ii) laboratory preparation of ammonia from ammonium chloride
solutions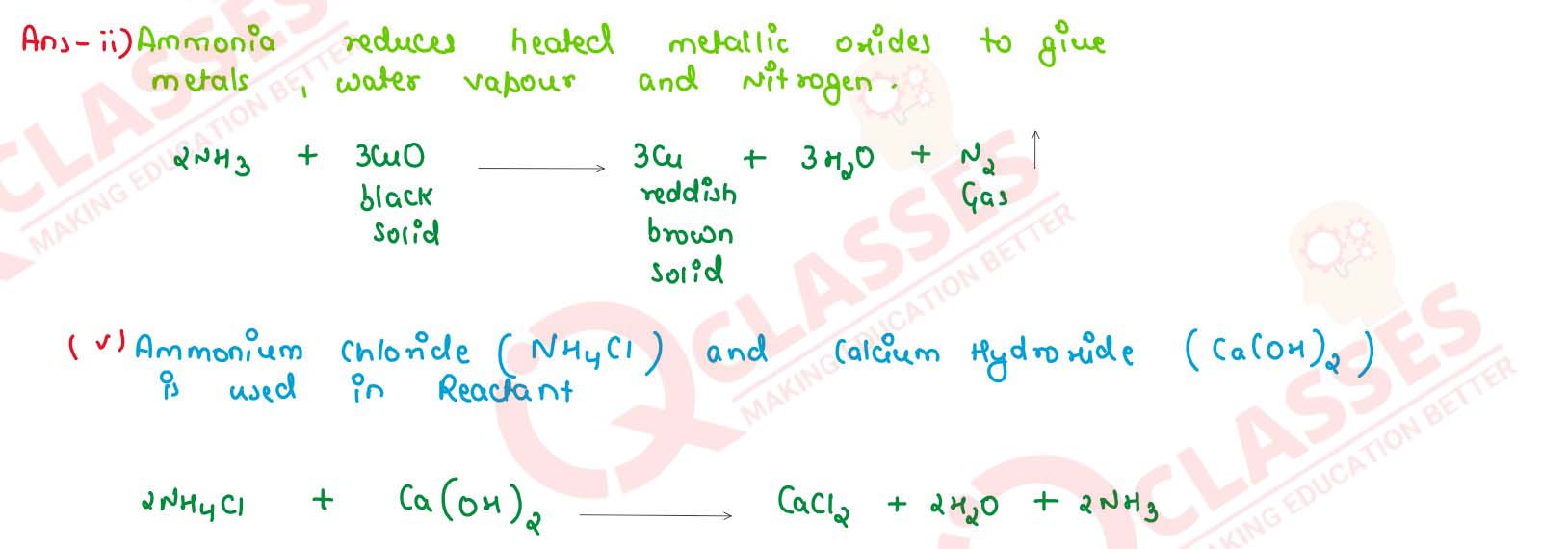
(i) reaction of ammonia with heated copper oxide
(ii) laboratory preparation of ammonia from ammonium chloride
solutions

2016
Q8
(a) Name the gas evolved in the following mixtures are heated
(i) Calcium hydroxide and ammonium chloride
(ii) Sodium nitrate and ammonium chloride
(b) Write balance chemical equation for each of the following:
(i) when excess of ammonia is treated with chlorine
(ii) An equation to illustrate the reducing nature of ammonia.
solutions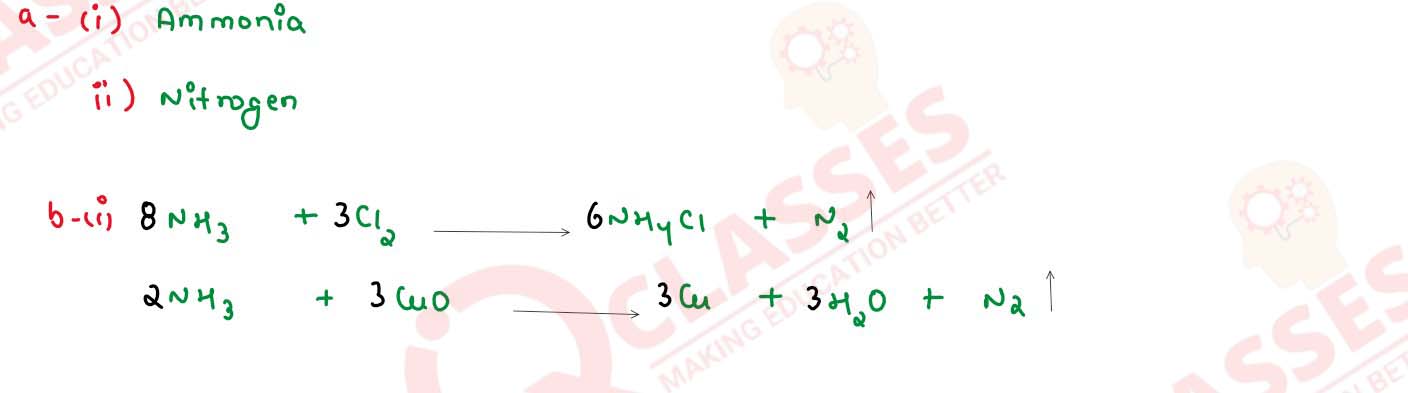
(i) Calcium hydroxide and ammonium chloride
(ii) Sodium nitrate and ammonium chloride
(b) Write balance chemical equation for each of the following:
(i) when excess of ammonia is treated with chlorine
(ii) An equation to illustrate the reducing nature of ammonia.
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment