Class 10 ICSE Analytical Chemistry Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Analytical Chemistry. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
class 10 ICSE Analytical Chemistry BoardQuestions
Analytical Chemistry BoardQuestions
2020
Q1
Identify the P,Q and R from the following observations:
Salt P has light bluish green colour.On heating,it produces a black coloured residue. Salt P produces brisk effervescence with dil. HCl and the gas evolved turns lime water milky, but no action with acidified potassium dichromate solution.
solutions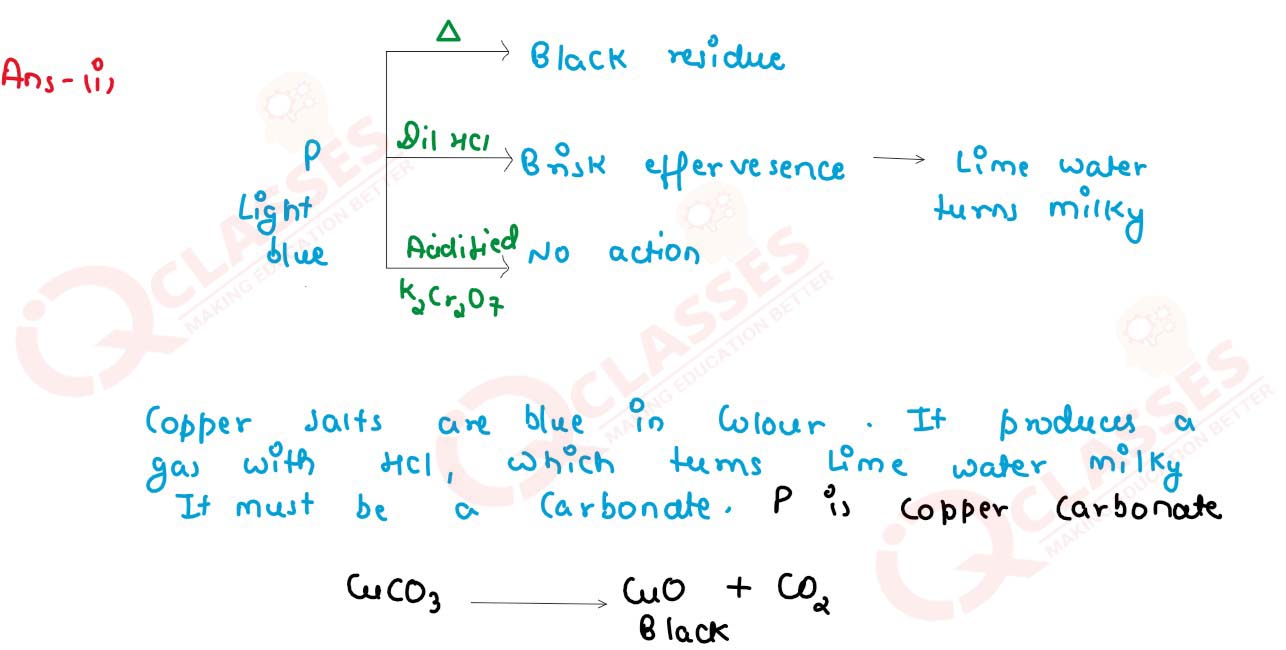
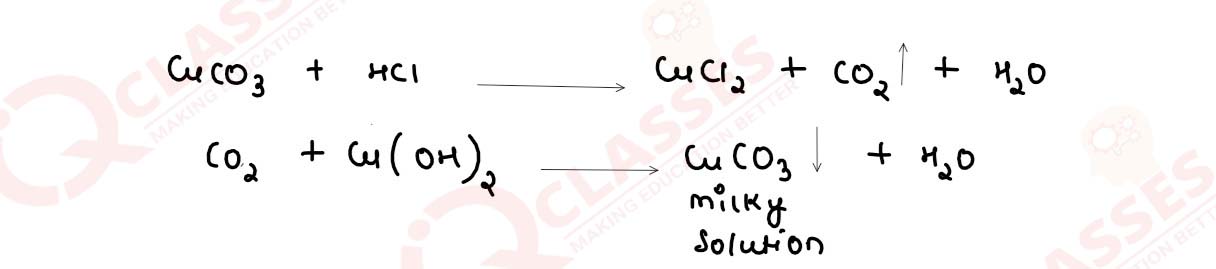
Salt P has light bluish green colour.On heating,it produces a black coloured residue. Salt P produces brisk effervescence with dil. HCl and the gas evolved turns lime water milky, but no action with acidified potassium dichromate solution.
solutions


2019
Q1
Copper sulphate solution reacts with sodium hydroxide solution to form a
precipitate of copper hydroxide according to the equation:
2NaOH + CuSO4 -> Na2SO4+ Cu(OH)2
(i) What mass of copper hydro,xide is precipitated by using 200 gm sodium hydroxide?
[H = 1, O= 16, Na=23, S=32, cu=64]
(ii) What is the colour of the precipitate formed?
solutions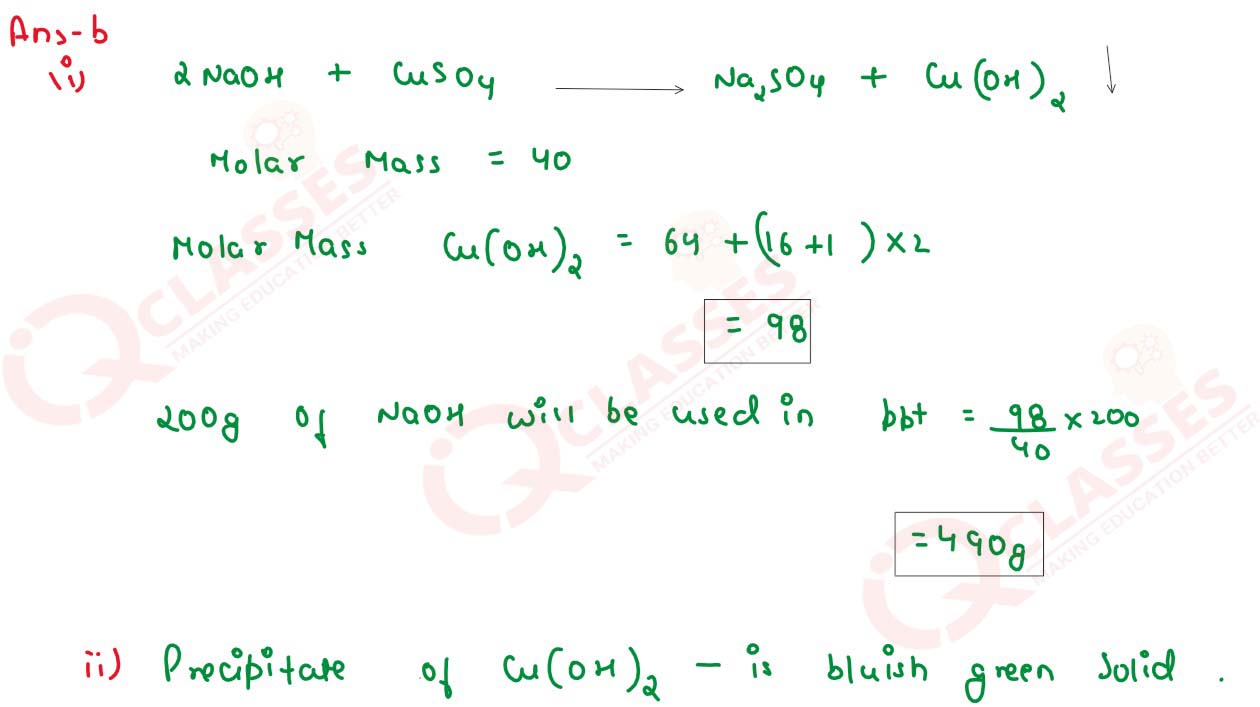
2NaOH + CuSO4 -> Na2SO4+ Cu(OH)2
(i) What mass of copper hydro,xide is precipitated by using 200 gm sodium hydroxide?
[H = 1, O= 16, Na=23, S=32, cu=64]
(ii) What is the colour of the precipitate formed?
solutions
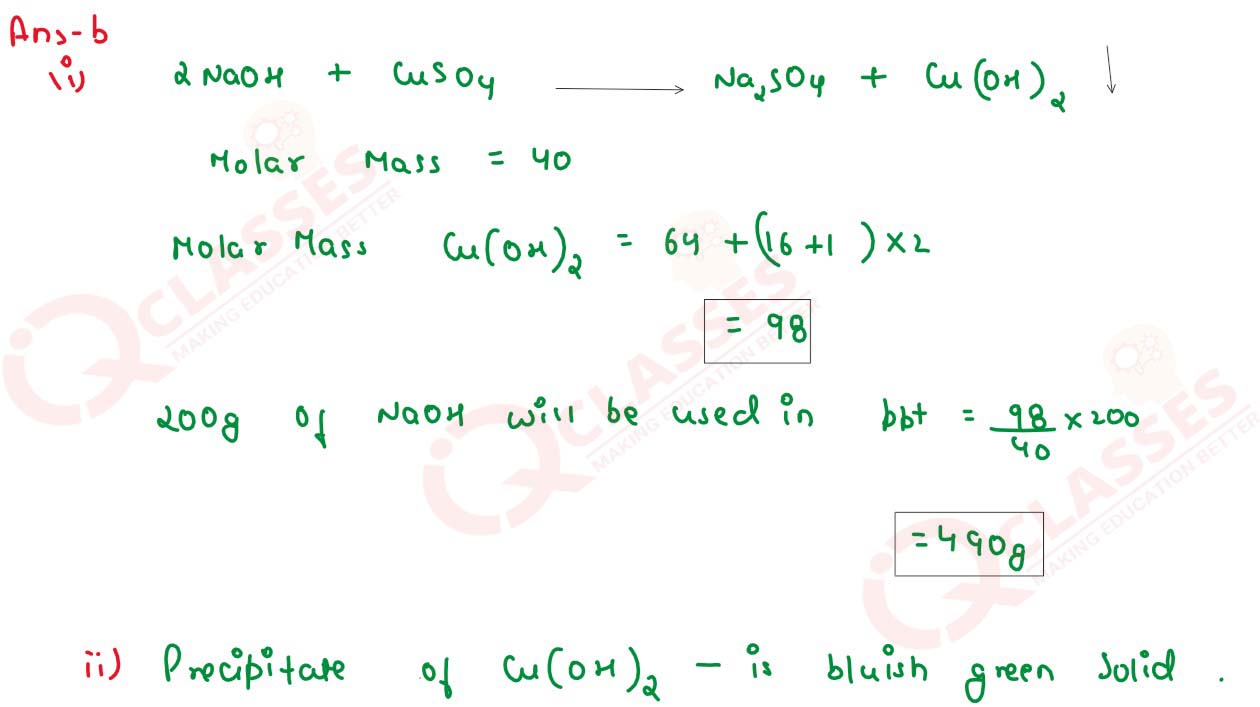
2018
Q1
Give a chemical test to distinguish between the following pairs of chemicals:
(i) Lead nitrate solution and Zinc nitrate solution
(ii) Sodium chloride solution and Sodium nitrate solution
solutions
(i) Lead nitrate solution and Zinc nitrate solution
(ii) Sodium chloride solution and Sodium nitrate solution
solutions

Q2
State one relevant observation for each of the following:
(i) Lead nitrate solution is treated with sodium hydroxide solution drop wise till it is in excess.
solutions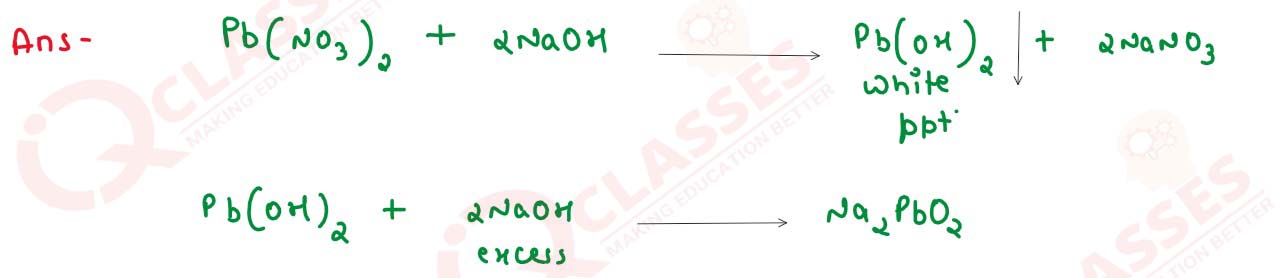
(i) Lead nitrate solution is treated with sodium hydroxide solution drop wise till it is in excess.
solutions

Q3
State one relevant observation for each Ofthe following:
Barium chloride solution is slowly added to sodium sulphate solution.
solutions
Barium chloride solution is slowly added to sodium sulphate solution.
solutions

2017
Q1
State one relevant observation for each of the following reactions:
Action of Sodium hydroxide solution on ferrous sulphate solution.
solutions
Action of Sodium hydroxide solution on ferrous sulphate solution.
solutions

Q2
Identify the salts P and Q from the observations given below.
(i) On performing the flame test salt P produces a lilac coloured flame and its solution gives a white precipitate with silver nitrate solution, which is soluble in Ammonium hydroxide solution.
(ii) When dilute HCl is added to a salt Q, a brisk effervescence is produced and the gas turns lime water milky. When NH4OH solution is added to the above mixture (after adding dilute HCI), it produces a white precipitate which is soluble in excess NH4OH solution.
solutions
(i) On performing the flame test salt P produces a lilac coloured flame and its solution gives a white precipitate with silver nitrate solution, which is soluble in Ammonium hydroxide solution.
(ii) When dilute HCl is added to a salt Q, a brisk effervescence is produced and the gas turns lime water milky. When NH4OH solution is added to the above mixture (after adding dilute HCI), it produces a white precipitate which is soluble in excess NH4OH solution.
solutions

2016
Q1
Identify the cations in each of the following case:
(i) NaOH solution when added to the Solution (A) gives a reddish brown precipitate.
(ii) NH4OH Solution when added to the Solution (B) gives white ppt which does not dissolve in excess.
(iii) NaOH Solution when added to Solution (C) gives white ppt which is insoluble in excess.
solutions
(i) NaOH solution when added to the Solution (A) gives a reddish brown precipitate.
(ii) NH4OH Solution when added to the Solution (B) gives white ppt which does not dissolve in excess.
(iii) NaOH Solution when added to Solution (C) gives white ppt which is insoluble in excess.
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment