Class 10 ICSE Analytical Chemistry Mostlikely QuestionBank
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Analytical Chemistry. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
class 10 ICSE Analytical Chemistry MostlikelyQuestionbank
Analytical Chemistry MostlikelyQuestionbank
Q1
Write balanced equations .
(a) Reaction of sodium hydroxide solution with Iron (Ill) chloride solution.
(b) Copper sulphate solution with ammonium hydroxide solution.
solutions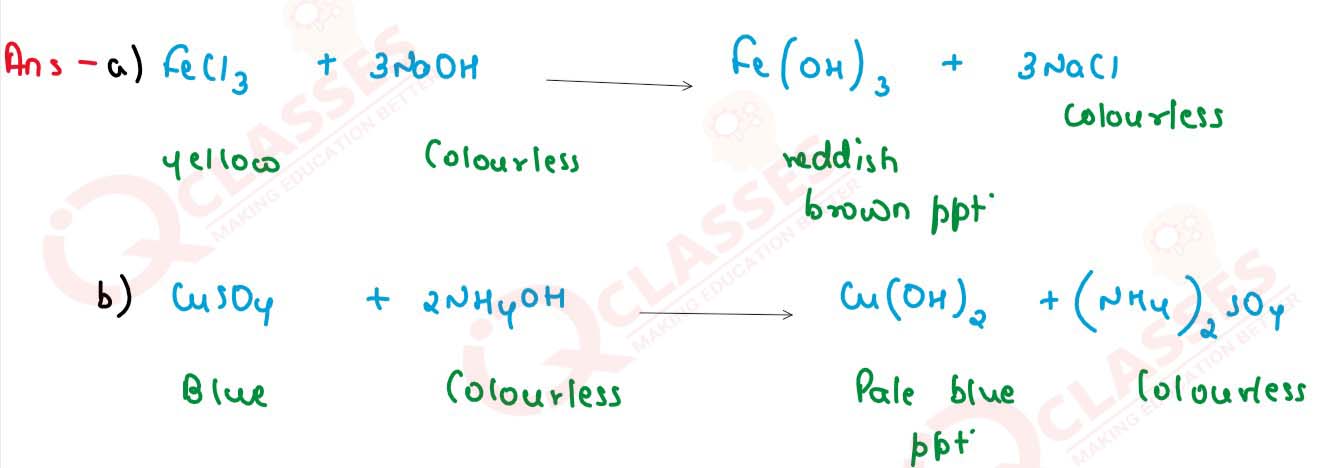
(a) Reaction of sodium hydroxide solution with Iron (Ill) chloride solution.
(b) Copper sulphate solution with ammonium hydroxide solution.
solutions

Q2
Name the probable cation present based on the
following observations
(a) White precipitate insoluble in NH4OH but soluble in NaOH
(b) Blue coloured solution
solutions
(a) White precipitate insoluble in NH4OH but soluble in NaOH
(b) Blue coloured solution
solutions

Q3
Write balanced equations for the following conversions
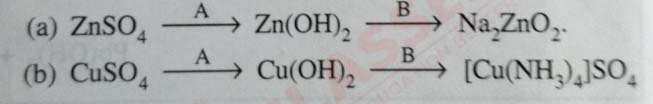
solutions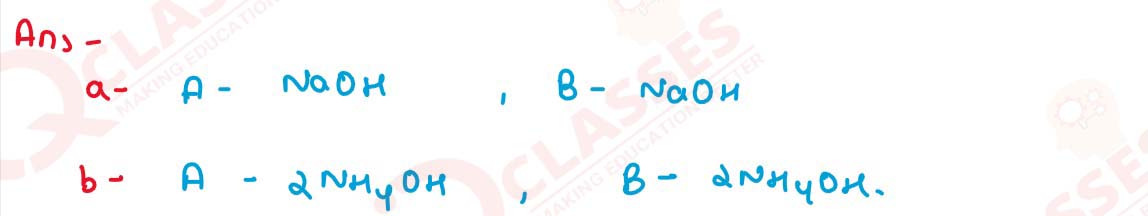
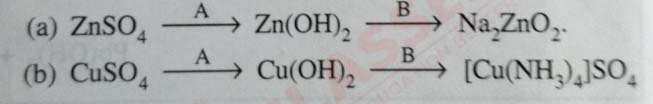
solutions

Q4
Name:
(a) a metallic hydroxide soluble in excess of NH4OH.
(b) a metallic oxide soluble in excess of caustic soda solution.
(c) a strong alkali.
(d) a weak alkali.
(e) two colourless metal ions.
(f) two coloured metal ions.
(g) a metal that evolves a gas which burns with a pop sound when boiled with alkali solutions.
(h) two bases which are not alkalis but dissolve in strong.
(j) a coloured rnetallic oxide which dissolves in alkalis to yield colourless solutions.
(j) a colourless cation not a representative element.
solutions

(a) a metallic hydroxide soluble in excess of NH4OH.
(b) a metallic oxide soluble in excess of caustic soda solution.
(c) a strong alkali.
(d) a weak alkali.
(e) two colourless metal ions.
(f) two coloured metal ions.
(g) a metal that evolves a gas which burns with a pop sound when boiled with alkali solutions.
(h) two bases which are not alkalis but dissolve in strong.
(j) a coloured rnetallic oxide which dissolves in alkalis to yield colourless solutions.
(j) a colourless cation not a representative element.
solutions


Q5
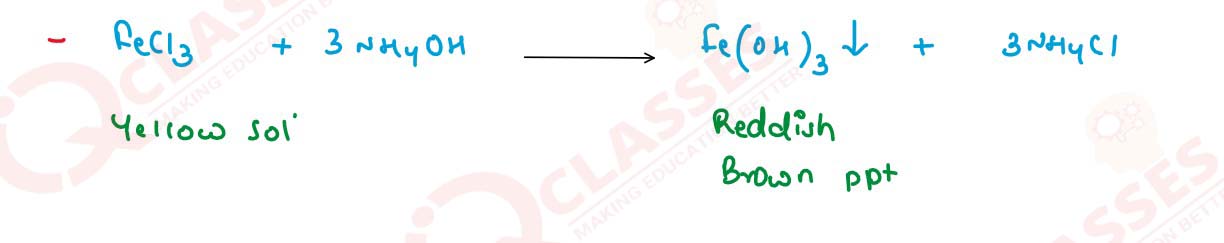
What happens when ammonia solution is added first
dropwise and then in excess to the following solutions :
(i) CuSO4
(ii) ZnSO4
(iii) FeCl3
Write balanced equations for these reactions.
solutions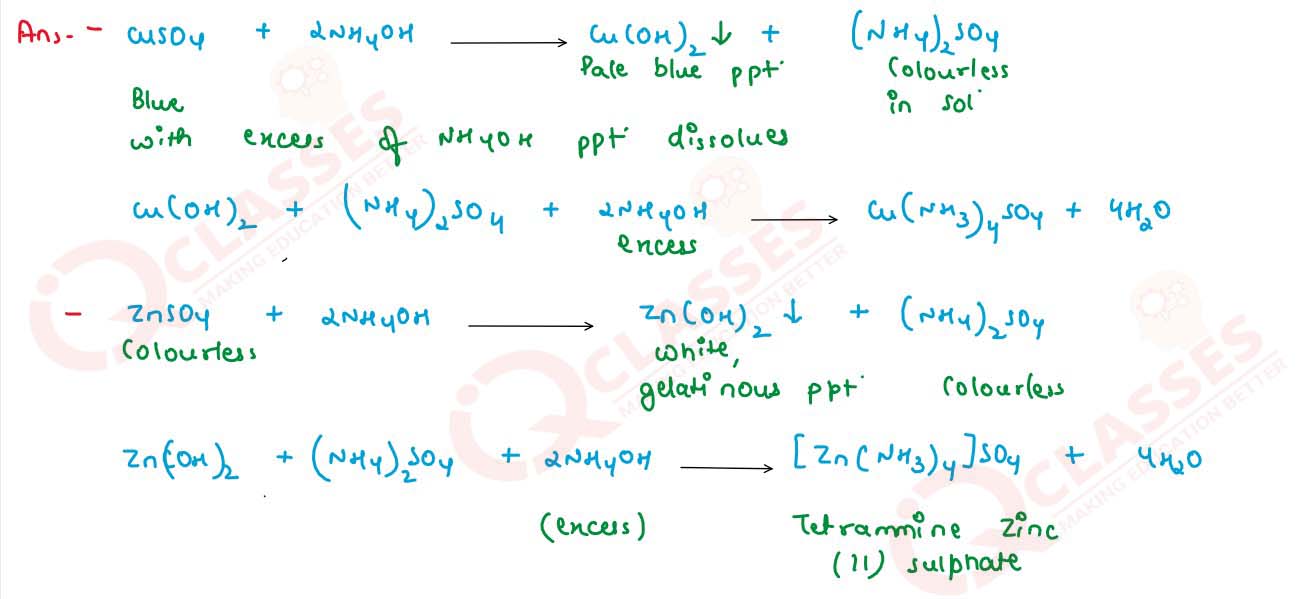
(i) CuSO4
(ii) ZnSO4
(iii) FeCl3
Write balanced equations for these reactions.
solutions



Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment