Class 10 Chemistry ICSE Chemical Bonding Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Chemical Bonding. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2019
Q1
The election dot structure of:
(i) Nitrogen molecule [N = 7]
(ii) Sodium chloride (Na = 11, Cl = 17)
(iii) Ammonium ion [N = 7, H = 1]
solutions Sodium chloride (Na = 11, Cl = 17)(iii) Ammonium ion [N = 7, H = 1]](class10chemimp/CB/b-icse/1.jpg)
 Sodium chloride (Na = 11, Cl = 17)(iii) Ammonium ion [N = 7, H = 1]](class10chemimp/CB/b-icse/1-1.jpg)
(i) Nitrogen molecule [N = 7]
(ii) Sodium chloride (Na = 11, Cl = 17)
(iii) Ammonium ion [N = 7, H = 1]
solutions
 Sodium chloride (Na = 11, Cl = 17)(iii) Ammonium ion [N = 7, H = 1]](class10chemimp/CB/b-icse/1.jpg)
 Sodium chloride (Na = 11, Cl = 17)(iii) Ammonium ion [N = 7, H = 1]](class10chemimp/CB/b-icse/1-1.jpg)
2018
Q2
Give a reason : Ionic compounds have high melting point.
solutions
solutions

Q3
(i) What do you understand by lone pair of electrons?
(ii) Draw the electron dot diagram of hydronium ion (H=1,O=8)
solutions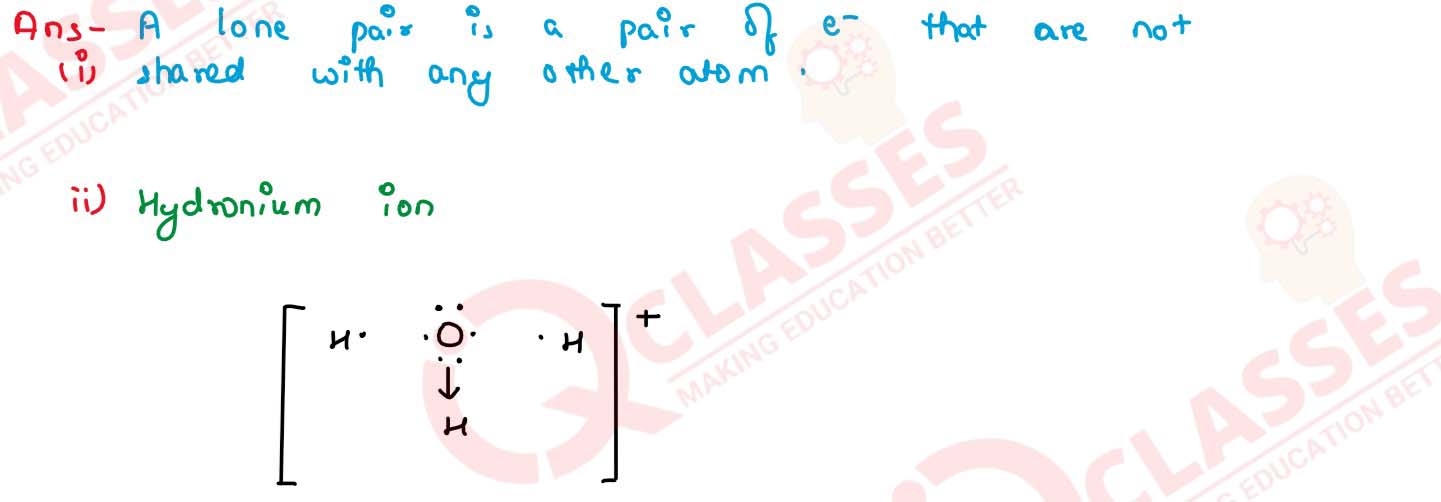
(ii) Draw the electron dot diagram of hydronium ion (H=1,O=8)
solutions

2017
Q4
State the type of bonding in the following molecules:
(i) Water
(ii) Calcium oxide
solutions
(i) Water
(ii) Calcium oxide
solutions

Q5
Draw an electron dot diagram to show the formation of each of the following compounds:
(a) Methane
(b) Magnesium Chloride
[H=1,C=6,Mg=12,Cl=17]
solutions![Draw an electron dot diagram to show the formation of each of the following compounds:
(a) Methane
(b) Magnesium Chloride
[H=1,C=6,Mg=12,Cl=17]](class10chemimp/CB/b-icse/5.jpg)
(a) Methane
(b) Magnesium Chloride
[H=1,C=6,Mg=12,Cl=17]
solutions
![Draw an electron dot diagram to show the formation of each of the following compounds:
(a) Methane
(b) Magnesium Chloride
[H=1,C=6,Mg=12,Cl=17]](class10chemimp/CB/b-icse/5.jpg)

Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment