Class 10 Chemistry ICSE Metallurgy Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Metallurgy. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
Sulphide ore is added to a tank containing oil and water, and then stirred or agitated with air.
solutions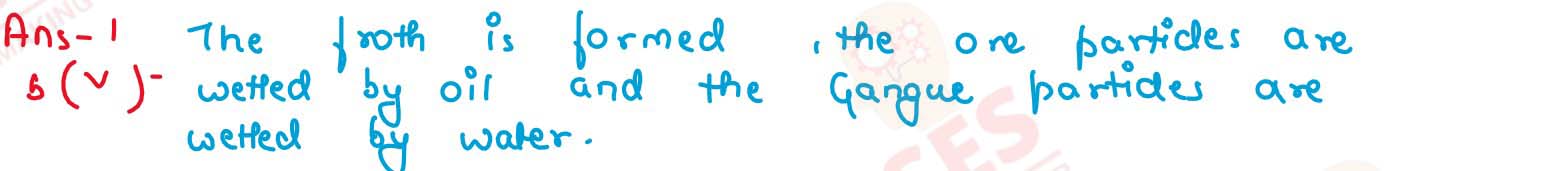

solutions
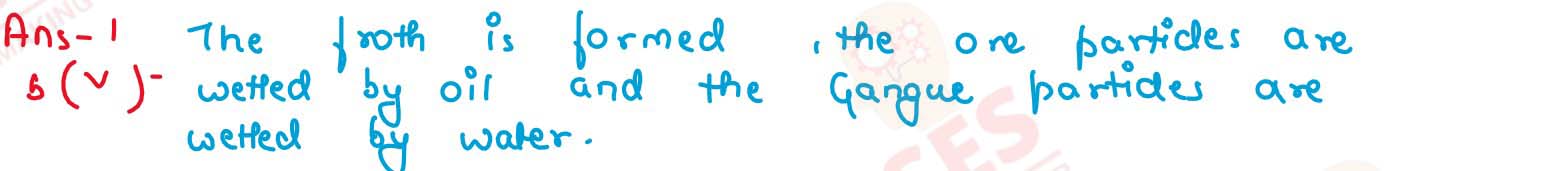

Q2
Bayer's process is used to concentrate bauxite ore to alumina give balance chemical equation for the
reaction taking place for its conversion from bauxite to alumina.
solutions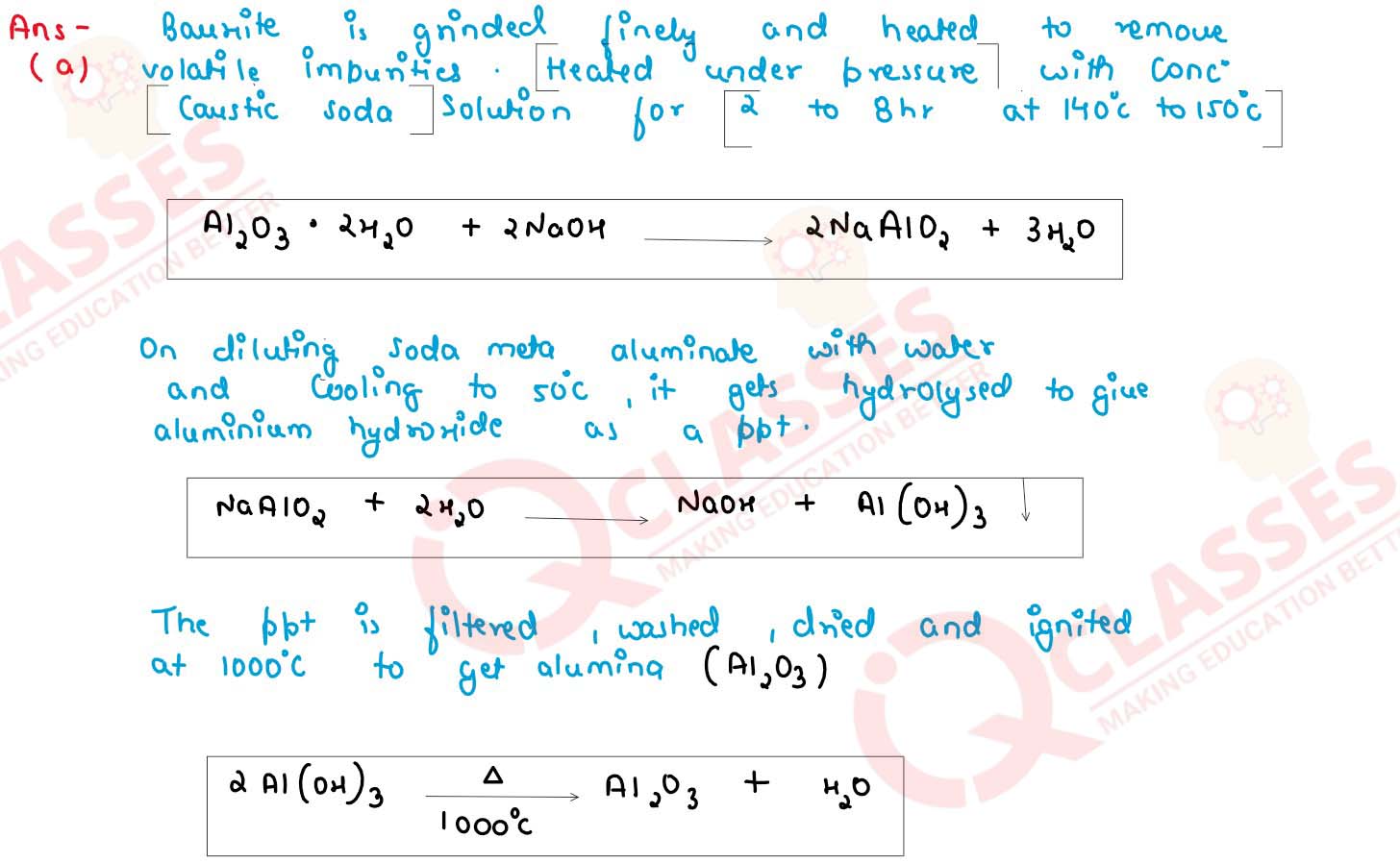
solutions

2019
Q3
Name the gas evolved in the following case:
Alumina undergoes electrolytic reduction.
solutions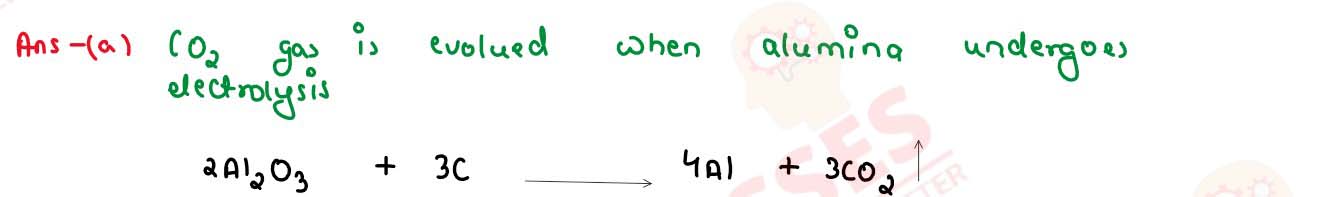
Alumina undergoes electrolytic reduction.
solutions

Q4
(a) Give the chemical formula of:
(i) Bauxite
(ii) Cryolite
(iii) Sodium alyminate
(b) Answer the following questions based on the extraction of aluminium from alumina by Hall-Heroult's Process.: (i) What is the function of cryolite used along with alumina as the electrolyte?
(ii) Why is powdered coke sprinkled on top of the electrolyte? (iii) Name the electrode, from which aluminium is collected.
solutions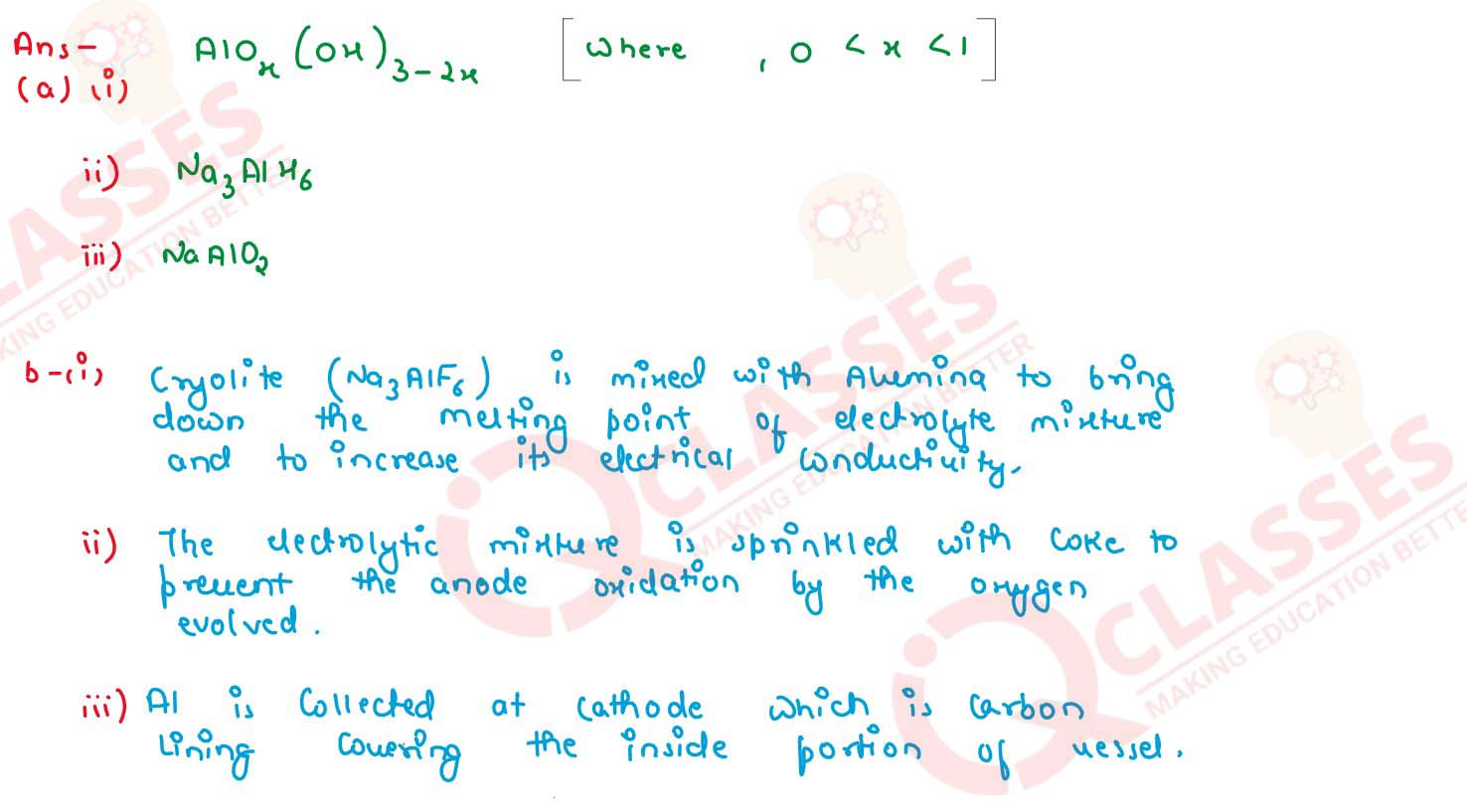
(i) Bauxite
(ii) Cryolite
(iii) Sodium alyminate
(b) Answer the following questions based on the extraction of aluminium from alumina by Hall-Heroult's Process.: (i) What is the function of cryolite used along with alumina as the electrolyte?
(ii) Why is powdered coke sprinkled on top of the electrolyte? (iii) Name the electrode, from which aluminium is collected.
solutions

2018
Q5
Name the main component of the following alloys:
(i) Brass ,
(ii) Duralumin
solutions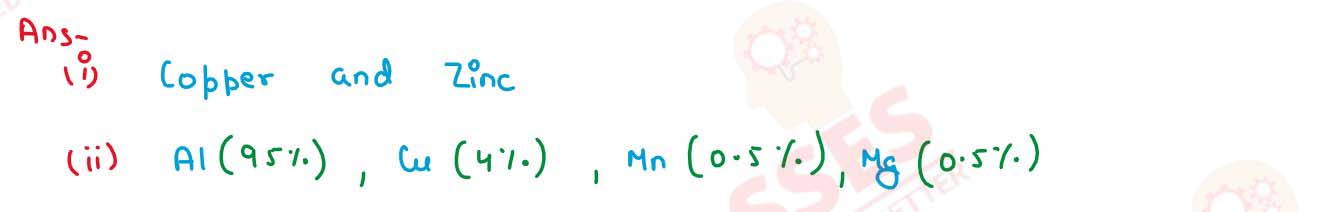
(i) Brass ,
(ii) Duralumin
solutions
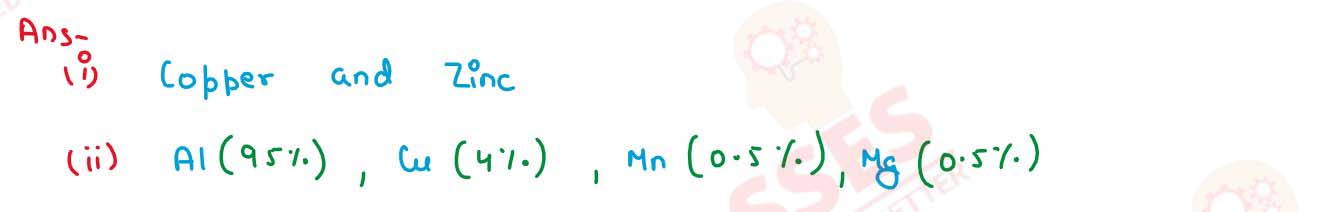
2017
Q6
Name the following:
(i) The process of coating of iron with zinc.
(ii) An alloy of lead and tin that is used in electrical circuits.
(iii) An ore of zinc containing its sulphide.
(iv) A metal oxide that can be reduced by hydrogen.
solutions
(i) The process of coating of iron with zinc.
(ii) An alloy of lead and tin that is used in electrical circuits.
(iii) An ore of zinc containing its sulphide.
(iv) A metal oxide that can be reduced by hydrogen.
solutions

Q7
Answer the following questions with respect to the electrolytic process in the extraction of
aluminum:
(i) Identify the components of the electrolyte other than pure alumina and the role played by each.
(ii) Explain why powdered coke is sprinkled over the electrolytic mixture.
solutions
(i) Identify the components of the electrolyte other than pure alumina and the role played by each.
(ii) Explain why powdered coke is sprinkled over the electrolytic mixture.
solutions

2016
Q8
(a) (i) Name the solution used to react with bauxite as a first step in obtaining pure aluminium
oxide in the Bayer's process.
(ii) Write the equation for the reaction where the aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide
(iii) Name the compound added to pure alumina to lower the fusion temperature during the electrolytic reduction of alumina
(iv) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
(v) Explain why it is preferable to use in number of graphite electrodes as anode instead of single electrode, during the above electrolysis.
solutions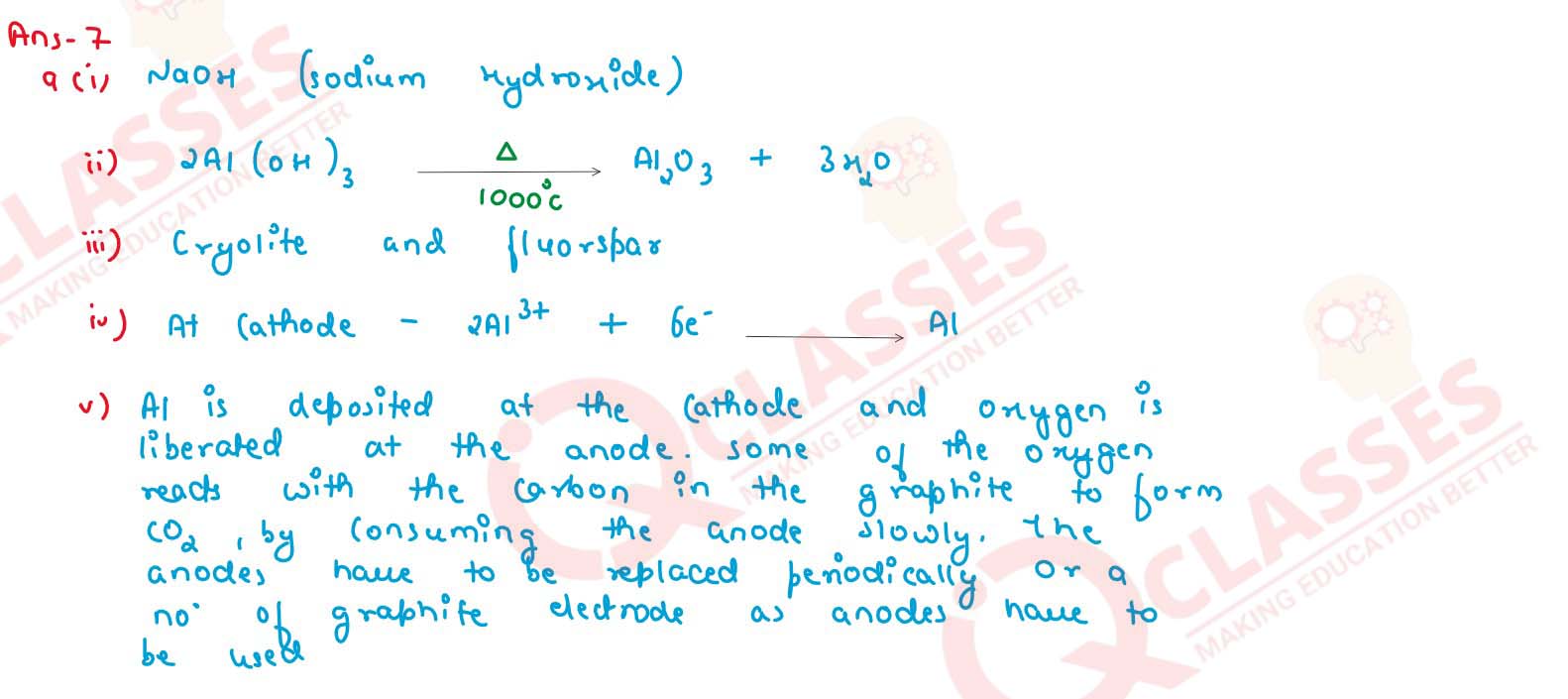
(ii) Write the equation for the reaction where the aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide
(iii) Name the compound added to pure alumina to lower the fusion temperature during the electrolytic reduction of alumina
(iv) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
(v) Explain why it is preferable to use in number of graphite electrodes as anode instead of single electrode, during the above electrolysis.
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment