Class 10 Chemistry ICSE Mole Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Mole. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
Calculate:
(i) The amount of each reactant required to produce 750ml of carbon dioxide,when two volumes of
carbon monoxide combine with one volume of oxygen to produce two volumes of carbon dioxide
2CO + O2 ---> 2CO2
(ii) The volume occupied by 80g of carbon dioxide at STP.
(iii) Calulate the number of molecules in 4.4gram of CO2 [Atomic mass of C=12,O=16]
(iv) State the law associated in question no.(i) above
solutions
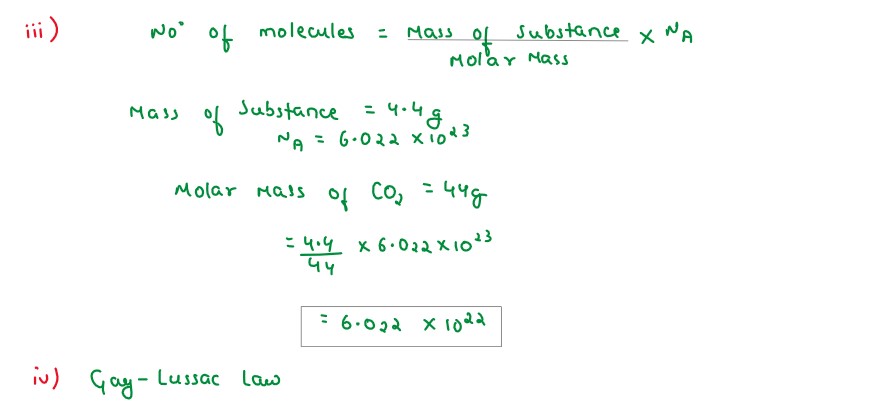
2CO + O2 ---> 2CO2
(ii) The volume occupied by 80g of carbon dioxide at STP.
(iii) Calulate the number of molecules in 4.4gram of CO2 [Atomic mass of C=12,O=16]
(iv) State the law associated in question no.(i) above
solutions

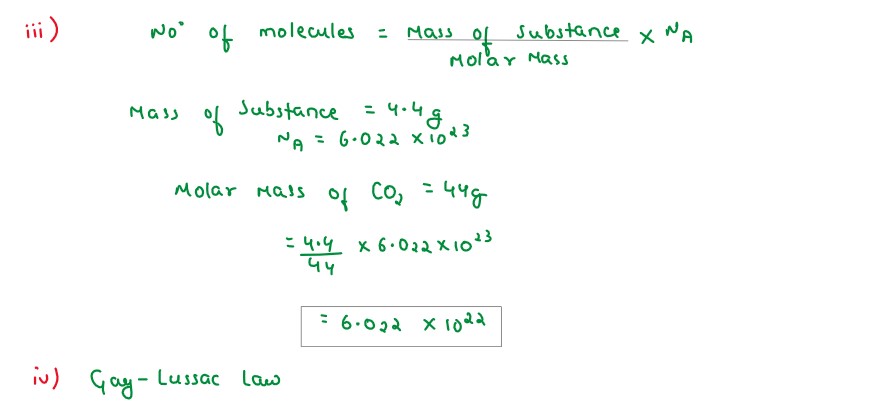
Q2
(i) State the volume occupied by 40gm of methane at STP,if its vapour density(V.D.) is 8
(ii) Calculate the number of moles present in 160gm of NaOH [Atomic mass : Na=23,H=1,O=16]
solutions
(ii) Calculate the number of moles present in 160gm of NaOH [Atomic mass : Na=23,H=1,O=16]
solutions

2019
Q1
Calculate:
(1) The number of moles in 12g of oxygen gas
(2) The weight of 1022 atoms of carbon.
[c=12,Avogadro's No.=6x 1023]
solutions
(1) The number of moles in 12g of oxygen gas
(2) The weight of 1022 atoms of carbon.
[c=12,Avogadro's No.=6x 1023]
solutions

Q2
Molcular formula of a compound is C6H18.Find its empirical formula
solutions
solutions

2018
Q1
(a) The percentage composition of a gas is:
Nitrogen 82.35% ,Hydrogen 17.64%
Find the empirical formula of the gas.[N=14,H=1]
(b) Aluminium carbide reacts with water according to the following equation
Al4C3 +12H2O --> 4Al(OH)3 + 3CH4
(i) What mass of Aluminium hydroxide is formed from 12g of Aluminium acbide?
(ii) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
(c) (i) If 150cc of gas A contains X molecules,how many molecules of gas B will be present in 75cc of B?
The gases A and B are under the same conditions of temperature and pressure
(ii) Name the law on which the above problem is based
solutions

Nitrogen 82.35% ,Hydrogen 17.64%
Find the empirical formula of the gas.[N=14,H=1]
(b) Aluminium carbide reacts with water according to the following equation
Al4C3 +12H2O --> 4Al(OH)3 + 3CH4
(i) What mass of Aluminium hydroxide is formed from 12g of Aluminium acbide?
(ii) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
(c) (i) If 150cc of gas A contains X molecules,how many molecules of gas B will be present in 75cc of B?
The gases A and B are under the same conditions of temperature and pressure
(ii) Name the law on which the above problem is based
solutions


2017
Q1
(i) Calculate the number of gram atoms in 4.6grams of sodium(Na=23)
(ii) Calculate the percentage of water of crystallization in CuSo4.5H2O
(H=1,O=16,S=32,Cu=64)
A compound of X and Y has the empirical formula XY2.Its vapour density is equal to its empirical formula weight.Determine its molecular formula
solutions
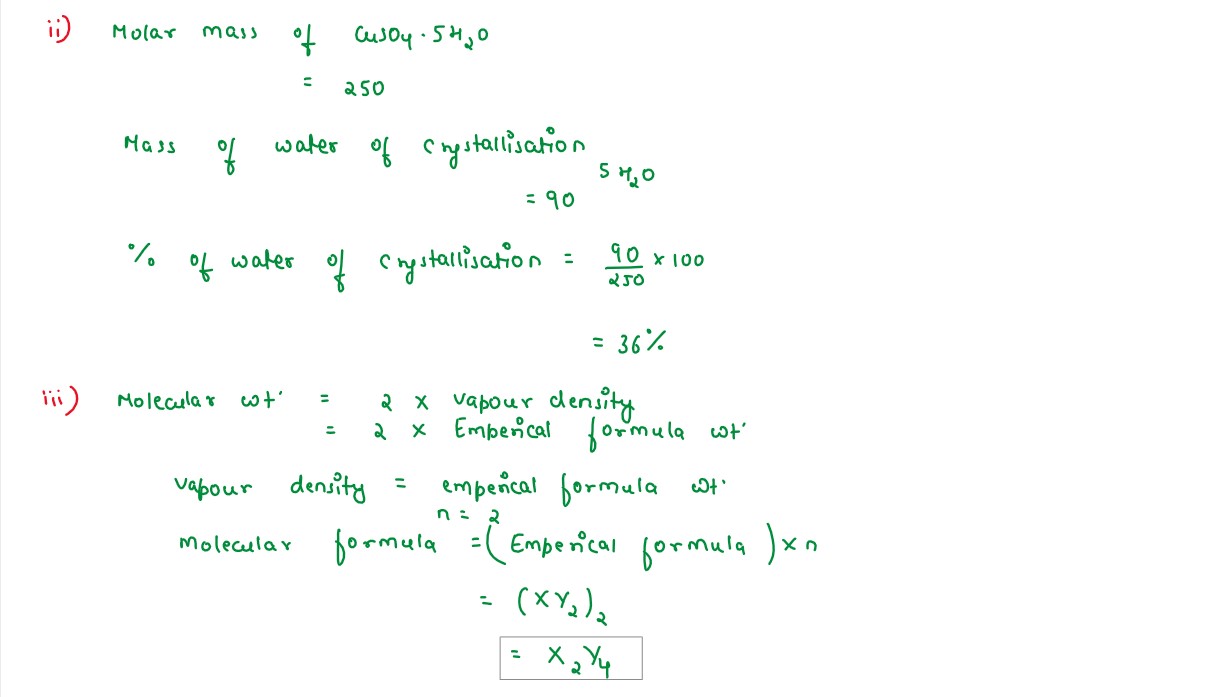
(ii) Calculate the percentage of water of crystallization in CuSo4.5H2O
(H=1,O=16,S=32,Cu=64)
A compound of X and Y has the empirical formula XY2.Its vapour density is equal to its empirical formula weight.Determine its molecular formula
solutions


2016
Q1
(a) A gas cylinder 12 x 1024 molecules of oxygen gas.
If Avogadro's number is 6 x1023;Calculate:
(i) the mass of oxygen present in the cylinder
(ii) the volume of oxygen at S.T.P. present in the cylinder.[O=16]
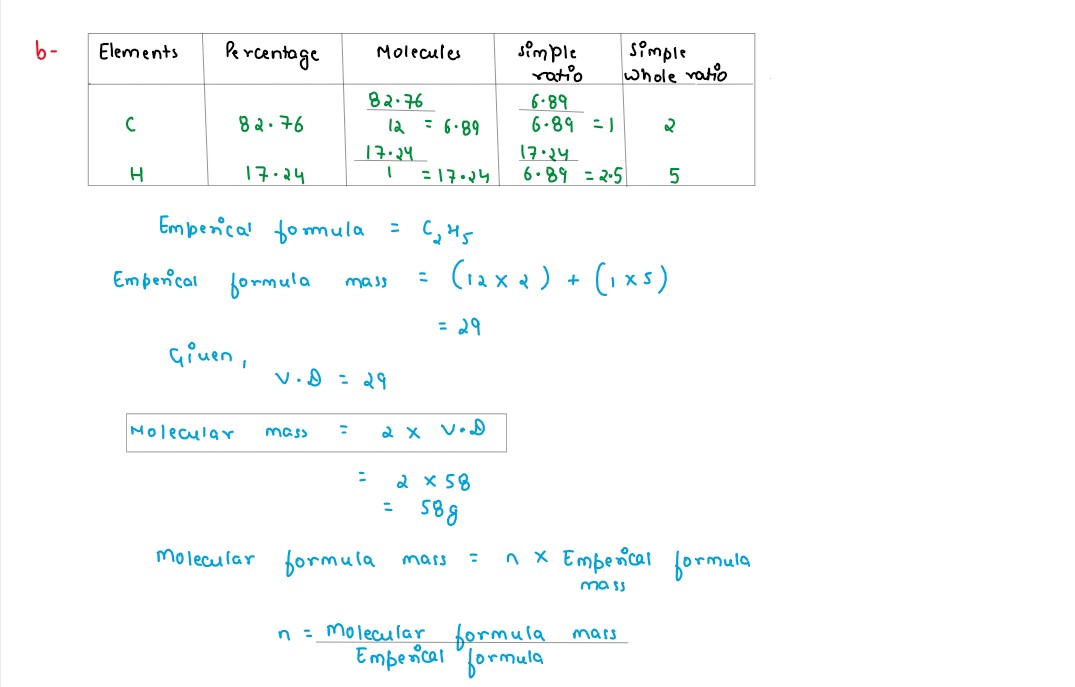
(b) A gaseous hydrocarbon contains 82.76% of carbon.Given that its vapour density is 29,find its molecular formula.[C=12,H=1]
(c) The equation 4NH3 + 5O2 -->4 NO + 6H2O, reresents the catalytic oxidation of ammonia.If 100cm3 of ammonia is used calculate the volume of oxygen required to oxidise the ammonia completely
solutions

If Avogadro's number is 6 x1023;Calculate:
(i) the mass of oxygen present in the cylinder
(ii) the volume of oxygen at S.T.P. present in the cylinder.[O=16]
(b) A gaseous hydrocarbon contains 82.76% of carbon.Given that its vapour density is 29,find its molecular formula.[C=12,H=1]
(c) The equation 4NH3 + 5O2 -->4 NO + 6H2O, reresents the catalytic oxidation of ammonia.If 100cm3 of ammonia is used calculate the volume of oxygen required to oxidise the ammonia completely
solutions




Add a comment