Class 10 Chemistry ICSE Periodic Properties Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Periodic Properties. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
Name the following elements :
(i) An alkaline earth metal present in Group 2 and period 3
(ii) A trivalent metal used to make light tools
(iii) A monovalent non metal present in fluorspar.
solutions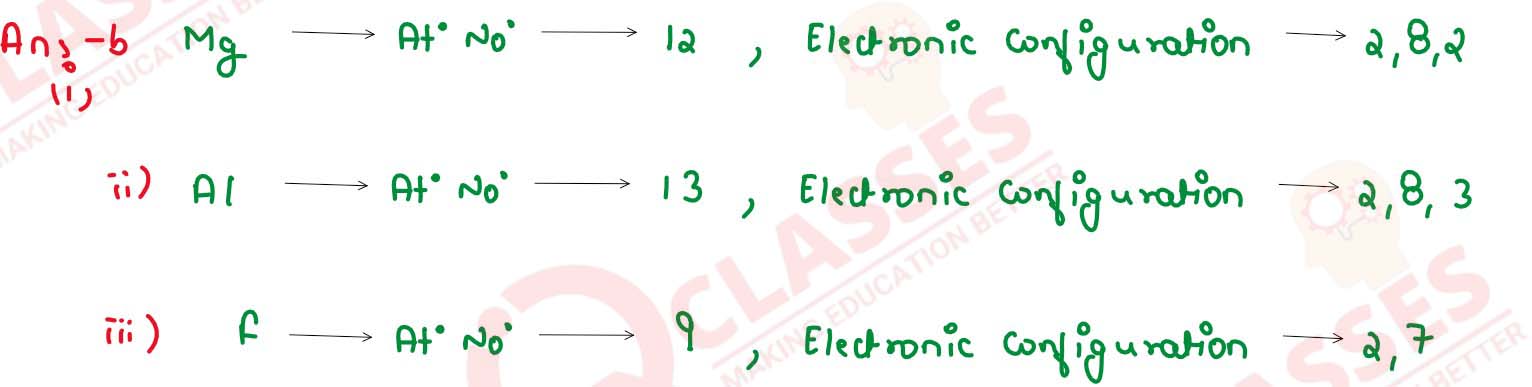
(i) An alkaline earth metal present in Group 2 and period 3
(ii) A trivalent metal used to make light tools
(iii) A monovalent non metal present in fluorspar.
solutions

2019
Q2
Arrange the following according to the instructions given in brackets:
(i) K, Pb, Ca, Zn (In the increasing order of the reactivity)
(ii) Mg2+, Cu2+, Na1+, H1+ (In the order of preferential discharge at the cathode)
(iii) Li, K, Na, H (In the decreasing order of their ionization potential)
(iv) F, B, N, O (In the increasing order of electron affinity)
solutions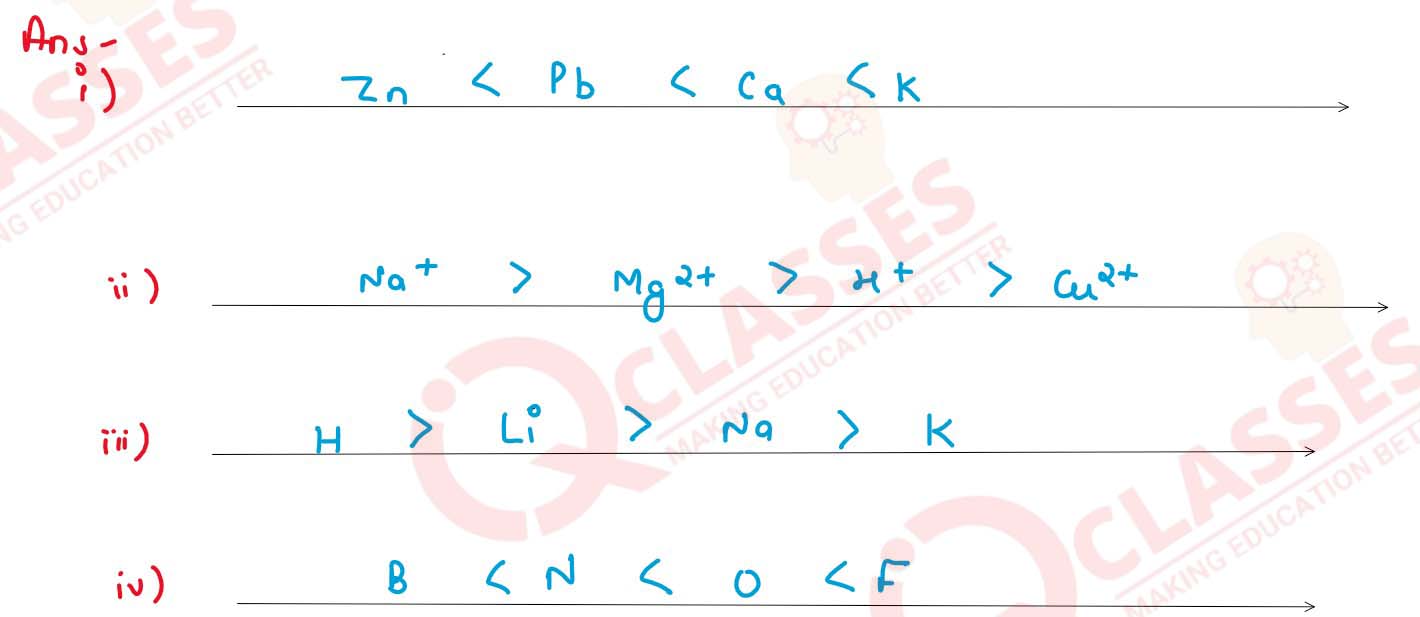
(i) K, Pb, Ca, Zn (In the increasing order of the reactivity)
(ii) Mg2+, Cu2+, Na1+, H1+ (In the order of preferential discharge at the cathode)
(iii) Li, K, Na, H (In the decreasing order of their ionization potential)
(iv) F, B, N, O (In the increasing order of electron affinity)
solutions

Q3
Study the extract of the periodic table given below answer the question that follow. Give the
alphabet corresponding to the element is question. DO NOT repeat an element.
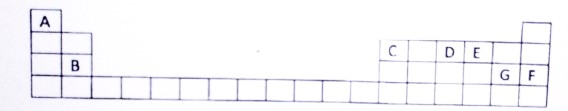
(i) Which element forms electrovalent compound with G
(ii) The ion of which element will migrate towards the cathode during electrolysis.
(iii) Which non metallic element has the valency of 2 ?
(iv) Which is an inert gas ?
solutions

(i) Which element forms electrovalent compound with G
(ii) The ion of which element will migrate towards the cathode during electrolysis.
(iii) Which non metallic element has the valency of 2 ?
(iv) Which is an inert gas ?
solutions

2018
Q4
(i) Inert gas do not form ions.
(ii) Ionisation potential increases across a period from left to right
(iii) Alkali metals are good reducing agents
solutions
(ii) Ionisation potential increases across a period from left to right
(iii) Alkali metals are good reducing agents
solutions

Q5
In Period 3 of the periodic table element B is placed to the left of element A. On the basis of the
information, choose the correct word from the brackets to complete the following statements :
(i) The element B would have (lower/higher) metallic character than A.
(ii) The element A would probably have (lesser/higher) electron affinity than B
(iii) The element A would have (greater/smaller) atomic size than B
solutions
(i) The element B would have (lower/higher) metallic character than A.
(ii) The element A would probably have (lesser/higher) electron affinity than B
(iii) The element A would have (greater/smaller) atomic size than B
solutions

2017
Q6
Match the atomic number 2,4,8,15 and 19 with each of the following:
(i) a solid non metal belonging to the 3rd period.
(ii) A metal of valency 1
(iii) A gaseous element with valency 2
(iv) an element belonging to group 2
(v) A rare gas.
solutions
(i) a solid non metal belonging to the 3rd period.
(ii) A metal of valency 1
(iii) A gaseous element with valency 2
(iv) an element belonging to group 2
(v) A rare gas.
solutions

Q7
Arrange the following as per the instruction given the brackets:
(i) He, Ar, Ne (Increasing order of the number of electron shells)
(ii) Na, Li, K (Increasing Ionisation Energy)
(iii) F, Cl, Br (Increasing electronegativity)
(iv) Na, K, I,i (Increasing atomic size)
solutions
(i) He, Ar, Ne (Increasing order of the number of electron shells)
(ii) Na, Li, K (Increasing Ionisation Energy)
(iii) F, Cl, Br (Increasing electronegativity)
(iv) Na, K, I,i (Increasing atomic size)
solutions

2016
Q8
Rewrite the following sentences by using the correct symbol > (greater than) or < (less than) in the
blanks given:
(1) The ionization potential of Potassium is.....that of Sodium.
(2) The electronegativity of Iodine is...... that of Chlorine.
solutions
(1) The ionization potential of Potassium is.....that of Sodium.
(2) The electronegativity of Iodine is...... that of Chlorine.
solutions

Q9
Use the letters only written in the Periodic Table given below to answer the questions that follow:
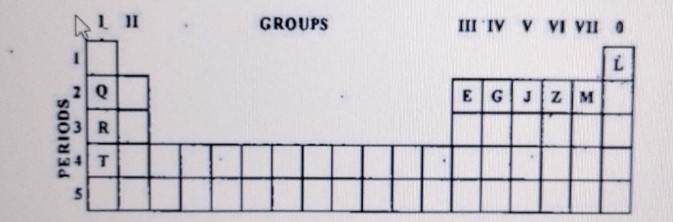
(i) State the number of valence electrons in atom J.
(ii) Which element shown forms ions with a single negative charge?
(iii) Which metallic element is more reactive than R?
(iv) Which element has its electrons arranged in four shells?
solutions

(i) State the number of valence electrons in atom J.
(ii) Which element shown forms ions with a single negative charge?
(iii) Which metallic element is more reactive than R?
(iv) Which element has its electrons arranged in four shells?
solutions

Q10
Fill in the blanks by %electing the correct word from the brackets.
(i) if an clement has a low ionization energy then it is likely to be............(metallic/non-metallic)
(ii) If an element has 7 electrons in its outer most shell then it is likely to have the........(largest/smallest) atomic size among all the elements in the same period.
solutions
(i) if an clement has a low ionization energy then it is likely to be............(metallic/non-metallic)
(ii) If an element has 7 electrons in its outer most shell then it is likely to have the........(largest/smallest) atomic size among all the elements in the same period.
solutions

Q11
The Following table shows the electronic configuration of the elements W, X, Y, Z:
Answer the following questions based on the table above:
(i) What type of Bond is formed between:
1. W and X
2. Y and Z
(ii) What is the formula of the compound formed between :
1. X and Z
2. W and X
solutions
Answer the following questions based on the table above:
(i) What type of Bond is formed between:
1. W and X
2. Y and Z
(ii) What is the formula of the compound formed between :
1. X and Z
2. W and X
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment