Class 10 Chemistry ICSE Acid, Bases and Salts Mostlikely QuestionBank
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter acid-base and salts. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
class 10 ICSE Acid, Bases and Salts Mostlikely QuestionBank
Acid, Bases and Salts Mostlikely-QuestionBank
Q1
Give two examples of each of the following :
(a) oxy-acid,
(b) hydracid,
(c) tribasic acid,
(d) dibasic acid.
solutions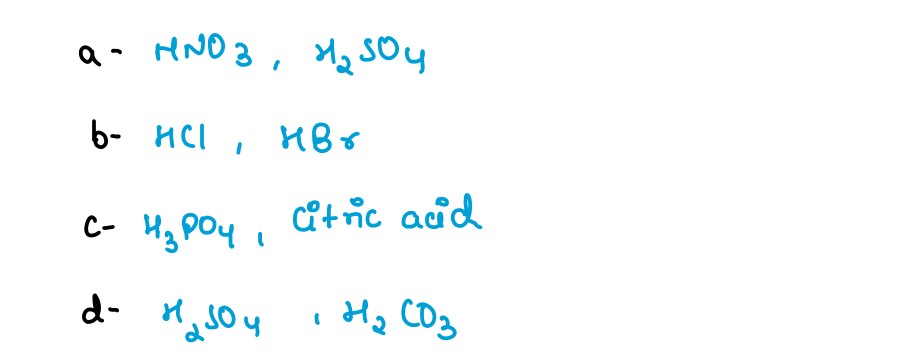
(a) oxy-acid,
(b) hydracid,
(c) tribasic acid,
(d) dibasic acid.
solutions
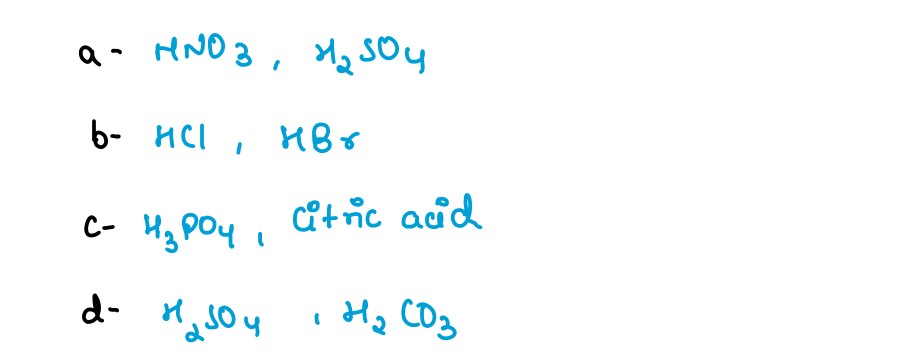
Q2
Explain the following :
(a) Carbonic acid gives an acid salt but hydrochloric add does not.
(b) Dil. HCl acid is stronger than highly concentrated acetic acid.
solutions
(a) Carbonic acid gives an acid salt but hydrochloric add does not.
(b) Dil. HCl acid is stronger than highly concentrated acetic acid.
solutions

Q3
(a) A solution has a pH of 7. Explain how you would
(i) increase its pH;
(ii) decrease its pH;
(b) If a solution changes the colour of litmus from red to blue, what can you say about its pH ?
(c) What can you say about the pH of a solution that liberates carbon dioxide from sodium carbonate.
solutions

(i) increase its pH;
(ii) decrease its pH;
(b) If a solution changes the colour of litmus from red to blue, what can you say about its pH ?
(c) What can you say about the pH of a solution that liberates carbon dioxide from sodium carbonate.
solutions


Q4
How would you prepare :
(a) copper sulphate crystals from a mixture of charcoal and black copper oxide,
(b) zinc sulphate crystals from zinc dust (powdered zinc and zinc oxide),
(c) sodium hydrogen carbonate crystals.
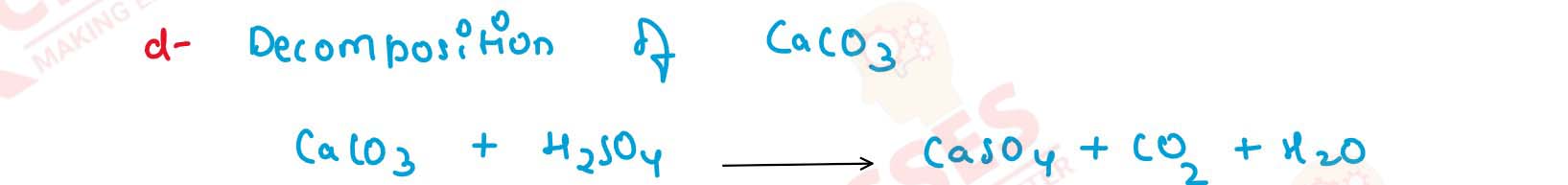
(d) Calcium sulphate from calcium carbonate.
solutions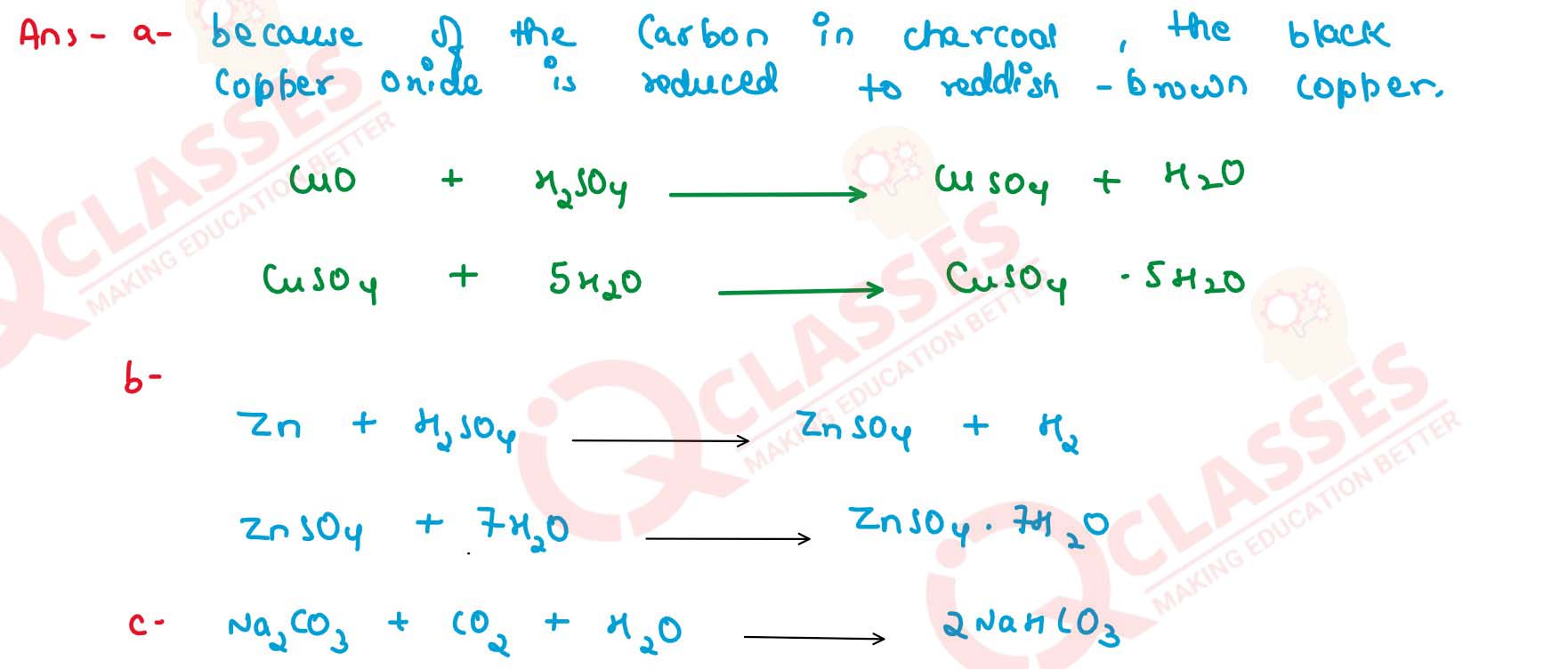
(a) copper sulphate crystals from a mixture of charcoal and black copper oxide,
(b) zinc sulphate crystals from zinc dust (powdered zinc and zinc oxide),
(c) sodium hydrogen carbonate crystals.
(d) Calcium sulphate from calcium carbonate.
solutions


Q5
The pH values of three solutions A, B and C are given in the table. Answer the following questions:
(i) Which solution will have no effect on litmus solution?
(ii) Which solution will liberate CO2 when reacted with sodium carbonate?
(iii) Which solution will turn red litmus solution blue?
solutions
| Solution | pH |
|---|---|
| A | 12 |
| B | 2 |
| C | 7 |
(ii) Which solution will liberate CO2 when reacted with sodium carbonate?
(iii) Which solution will turn red litmus solution blue?
solutions

Q6
(a) Fill in the blank from the choices given The basicity of acetic acid is ....... [3, 1, 4].
(b) Draw the structure of the stable positive ion formed when an acid dissolves in water.
(c) State the inference drawn from the Salt S is prepared by reacting dilute with copper oxide. Identify S.
(d) Give balanced chemical equations for the preparation of the following salts :
(i) Lead sulphate — from lead carbonate.
(ii) Sodium sulphate — using dilute sulphuric aicd.
(iii) Copper chloride — using copper carbonate.
solutions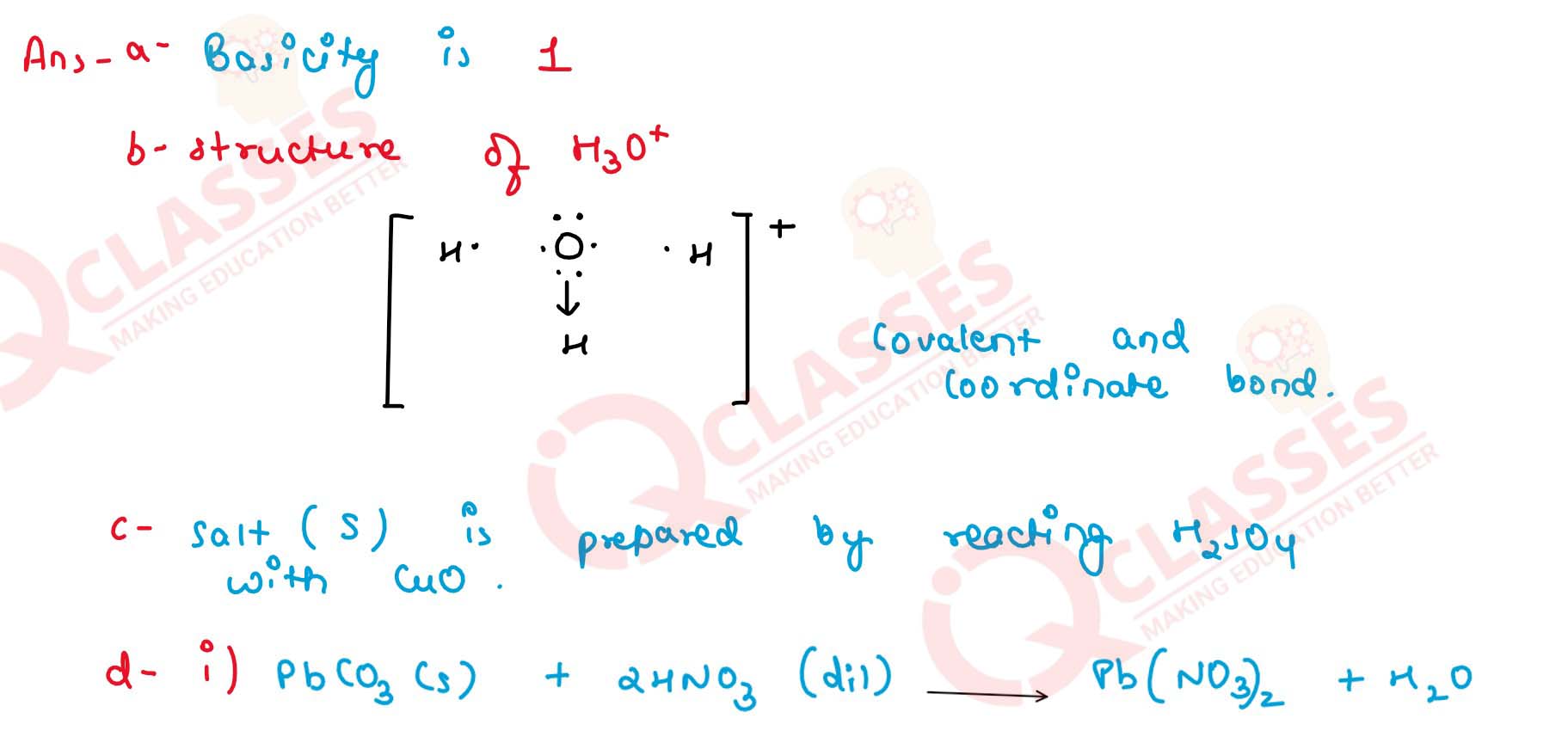
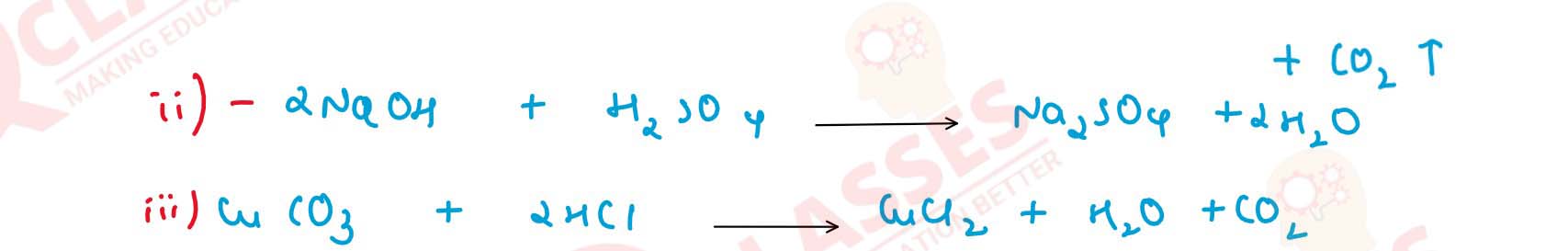
(b) Draw the structure of the stable positive ion formed when an acid dissolves in water.
(c) State the inference drawn from the Salt S is prepared by reacting dilute with copper oxide. Identify S.
(d) Give balanced chemical equations for the preparation of the following salts :
(i) Lead sulphate — from lead carbonate.
(ii) Sodium sulphate — using dilute sulphuric aicd.
(iii) Copper chloride — using copper carbonate.
solutions

Q7
Name the salt which on hydrolysis forms
(a) acidic
(b) basic and
(c) neutral solution.
Give a balanced equation for each reaction
solutions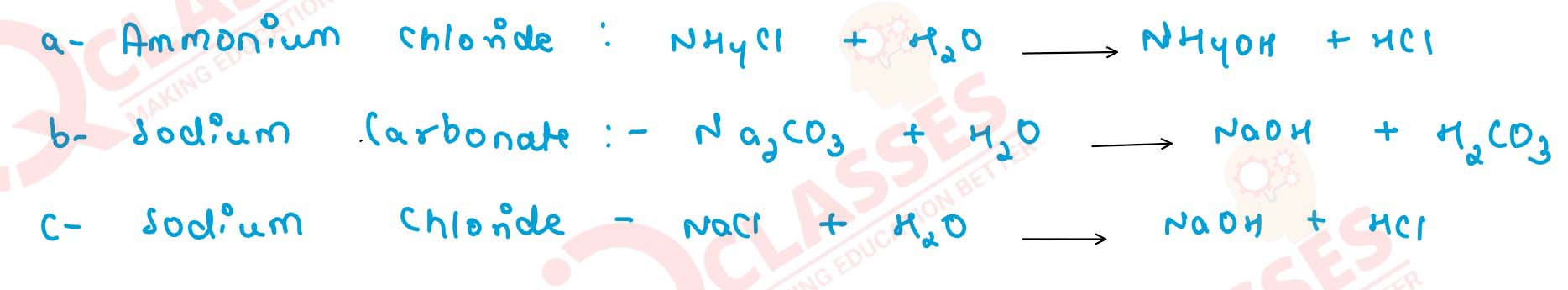
(a) acidic
(b) basic and
(c) neutral solution.
Give a balanced equation for each reaction
solutions
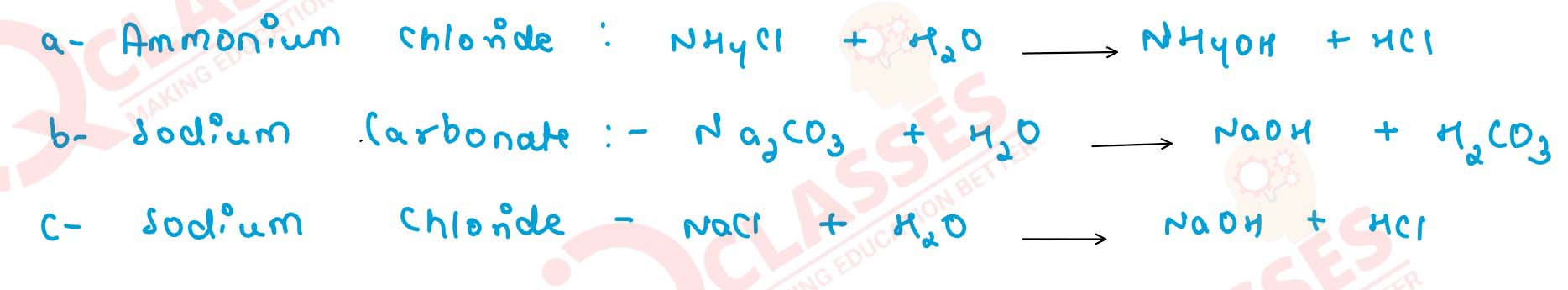

Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment