Class 10 Chemistry ICSE Nitric Acid Mostlikely QuestionBank
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Nitric Acid. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
class 10 ICSE Nitric Acid Mostlikely QuestionBank
Nitric Acid Mostlikely-QuestionBank
Q1
(a) Write a balanced chemical equation for the laboratory preparation of nitric acid.
(b) In the preparation of nitric acid from KNO3, concentrated hydrochloric acid is not used in place of concentrated sulphuric acid. Explain why ?
(c) Conc. nitric acid prepared in laboratory is yellow in colour. Why ? How is this colour removed ?
(d) Give reasons for the following : In the laboratory preparation of nitric acid, the mixture of concentrated sulphuric acid and sodium nitrate should not be heated very strongly above 200oC.
solutions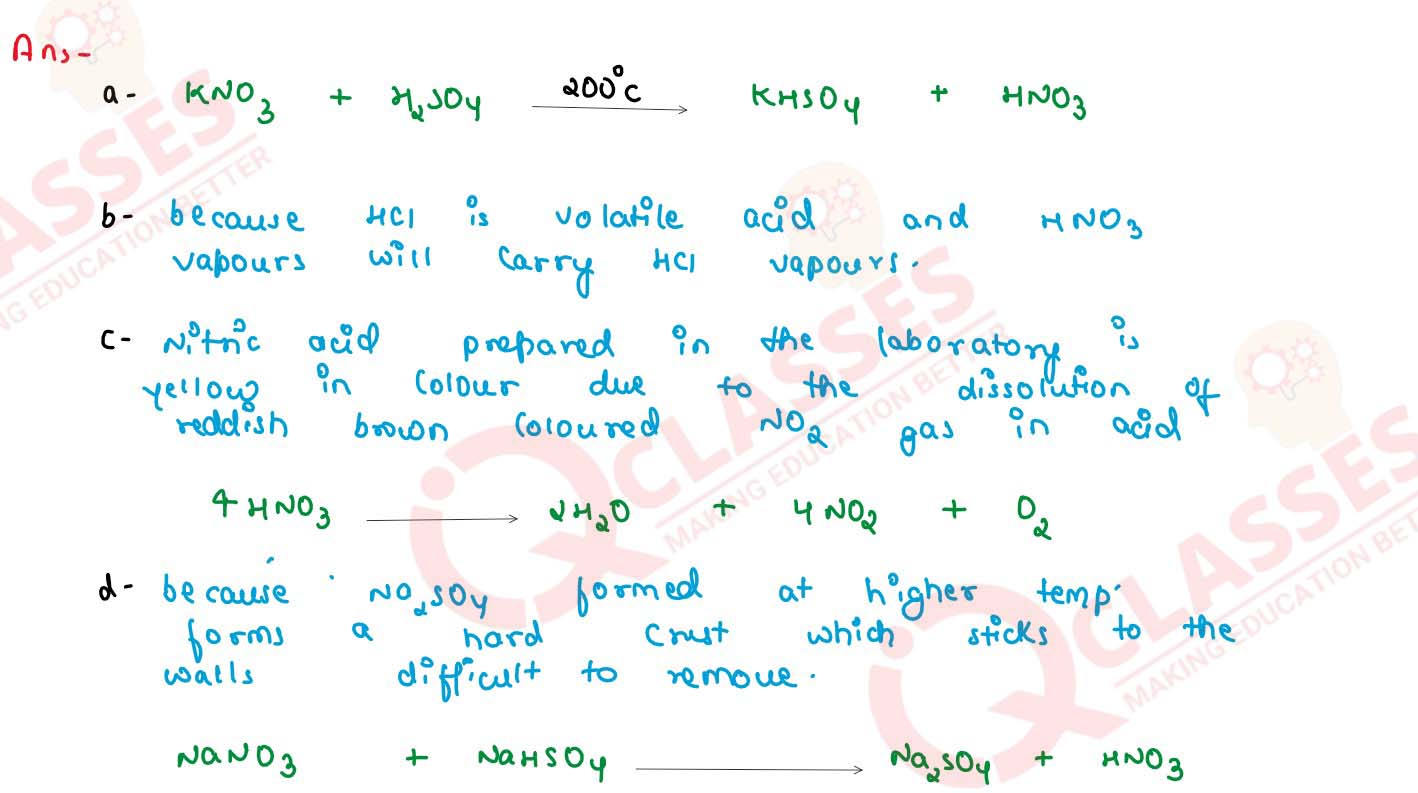
(b) In the preparation of nitric acid from KNO3, concentrated hydrochloric acid is not used in place of concentrated sulphuric acid. Explain why ?
(c) Conc. nitric acid prepared in laboratory is yellow in colour. Why ? How is this colour removed ?
(d) Give reasons for the following : In the laboratory preparation of nitric acid, the mixture of concentrated sulphuric acid and sodium nitrate should not be heated very strongly above 200oC.
solutions

Q2
Give two chemical equations for each of the following :
(a) Reactions of nitric acid with non-metals.
(b) Nitric acid showing as acidic character.
(c) Nitric acid acting as oxidising agent.
solutions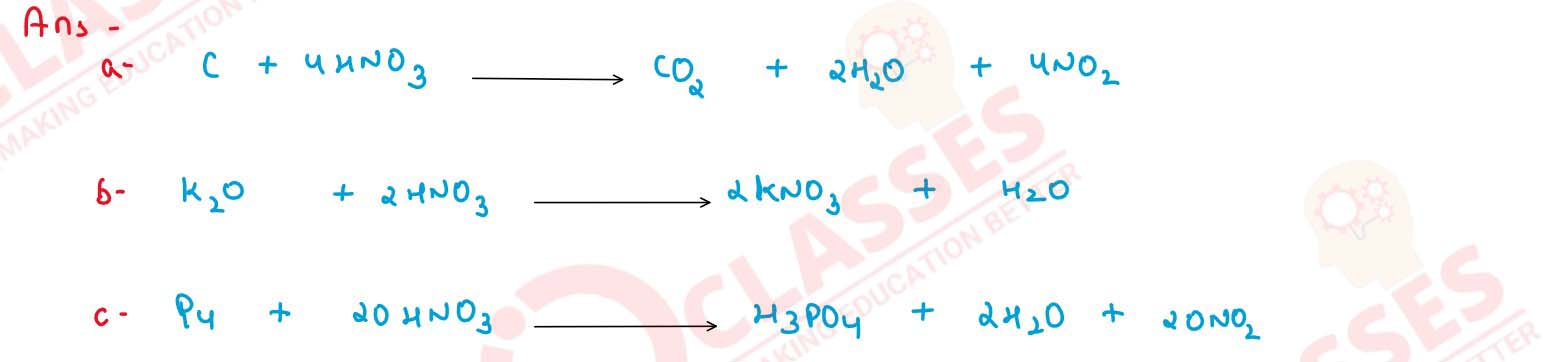
(a) Reactions of nitric acid with non-metals.
(b) Nitric acid showing as acidic character.
(c) Nitric acid acting as oxidising agent.
solutions

Q3
(i) What is the type of salt formed when the reactants are heated at a suitable temperature for the
preparation of Nitric acid?
(ii) State why for the preparation of Nitric acid, the complete apparatus is made up of glass.
solutions
solutions

Q4
(a) Name — the gas produced when copper reacts with conc. HNO3.
(b) State observation — zinc nitrate crystals are strongly heated.
(c) Correct the statement : Magnesium reacts with nitric acid to liberate hydrogen gas.
(d) Iron is rendered passive with fuming HNO3. Give reason.
(e) Give balanced equation for dilute nitric acid and copper carbonate.
solutions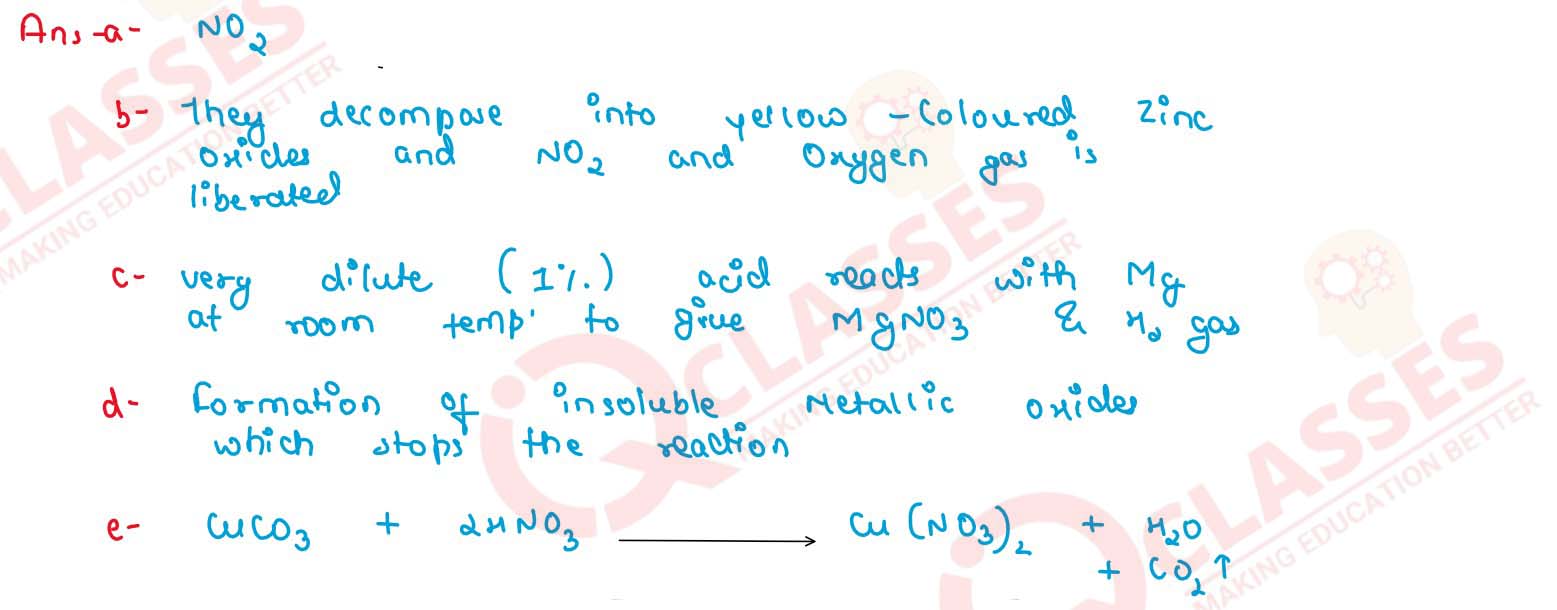
(b) State observation — zinc nitrate crystals are strongly heated.
(c) Correct the statement : Magnesium reacts with nitric acid to liberate hydrogen gas.
(d) Iron is rendered passive with fuming HNO3. Give reason.
(e) Give balanced equation for dilute nitric acid and copper carbonate.
solutions

Q5
Choose the correct answer :
(a) The nitrate salt which does not give a mixture of NO2 and 0, on heating is :
(i) AgNO3,
(ii) KNO3,
(iii) Cu(NO3), (iv) Zn(NO3)2.
(b) The chemical used in the brown ring test is :
(i) CuSO4,
(ii) FeSO4,
(iii) Fe,(SO4)3.
(iv) ZnSO4.
(c) Lead nitrate decomposes on heating to give : (i) NO
(ii) N20,
(iii) NO2,
(iv) N205.
solutions
(a) The nitrate salt which does not give a mixture of NO2 and 0, on heating is :
(i) AgNO3,
(ii) KNO3,
(iii) Cu(NO3), (iv) Zn(NO3)2.
(b) The chemical used in the brown ring test is :
(i) CuSO4,
(ii) FeSO4,
(iii) Fe,(SO4)3.
(iv) ZnSO4.
(c) Lead nitrate decomposes on heating to give : (i) NO
(ii) N20,
(iii) NO2,
(iv) N205.
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment