Class 10 ICSE H2SO4 Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter H2SO4. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
The acid which can produce carbon from cane sugar,is:
A. Concentrated Hydrochloric Acid
B. Concentrated Nitric Acid
C. Concentrated Sulphuric Acid
D. Concentrated Acetic Acid
solutions
A. Concentrated Hydrochloric Acid
B. Concentrated Nitric Acid
C. Concentrated Sulphuric Acid
D. Concentrated Acetic Acid
solutions

2019
Q1
Write a balanced chemical equation for each of the following reactions:
(ii) Action of dilute sulphuric acid on sodium hydroxide.
(iii) Action of dilute sulphuric acid on zinc sulphide.
solutions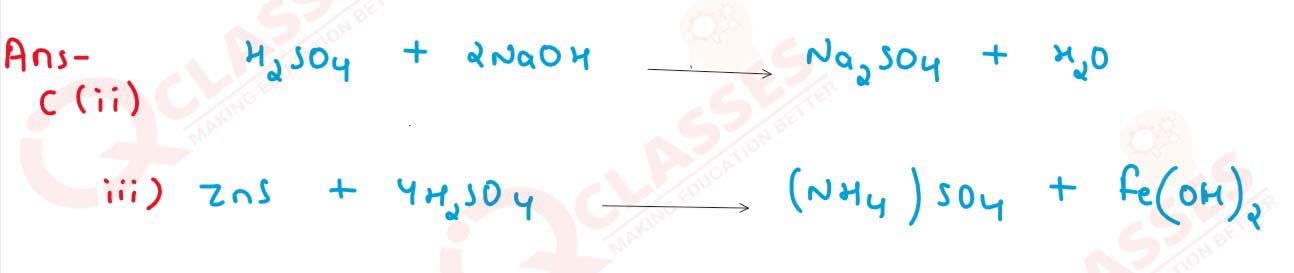
(ii) Action of dilute sulphuric acid on sodium hydroxide.
(iii) Action of dilute sulphuric acid on zinc sulphide.
solutions

2018
Q1
Which property of sulphuric acid is shown by the reaction of concentrated
sulphuric acid with:
(i) Ethanol?
(ii) Carbon?
solutions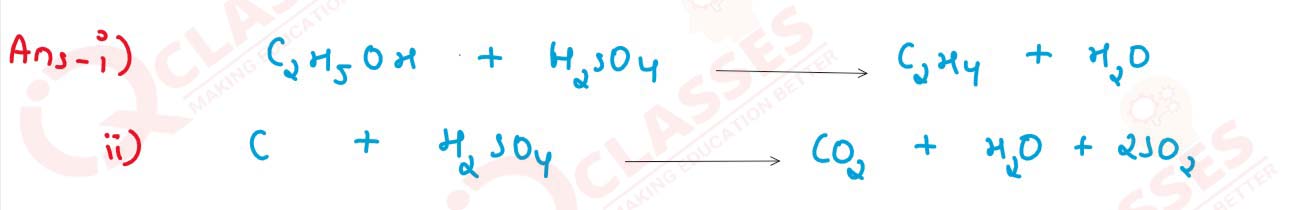
(i) Ethanol?
(ii) Carbon?
solutions

2017
Q1
(b) Write balanced chemical equations to show:
(i) The oxidizing action of conc. Sulphuric acid on Carbon.
(ii) The behavior of H2SO4 as an acid when it reacts with Magnesium.
(iii) The dehydrating property of conc. Sulphuric acid with sugar.
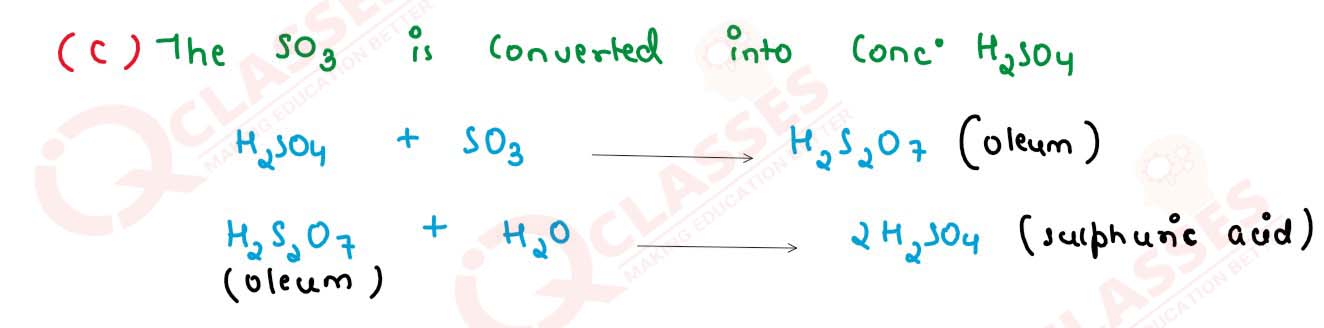
(c) Write balanced chemical equations to show how S03 is converted to Sulphuric acid in the contact process.
solutions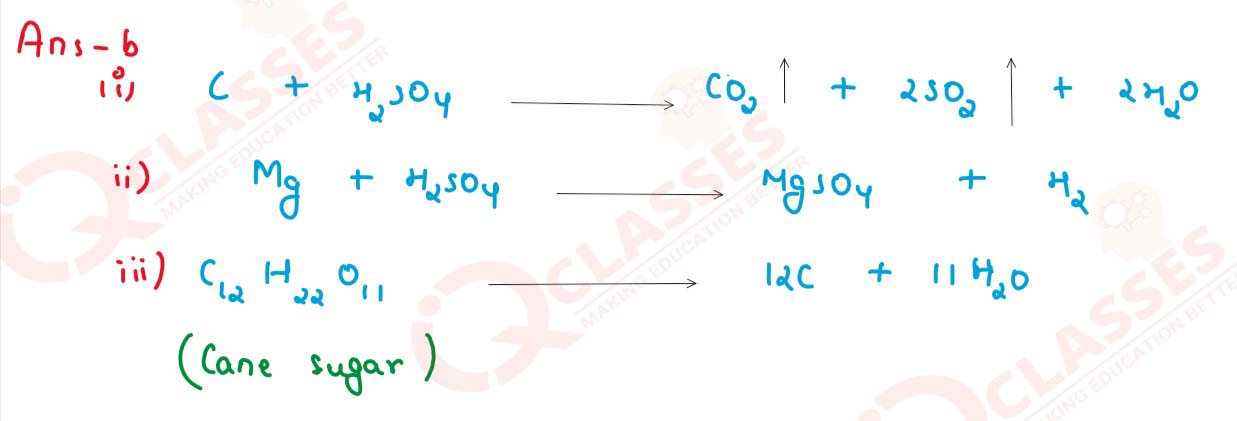
(i) The oxidizing action of conc. Sulphuric acid on Carbon.
(ii) The behavior of H2SO4 as an acid when it reacts with Magnesium.
(iii) The dehydrating property of conc. Sulphuric acid with sugar.
(c) Write balanced chemical equations to show how S03 is converted to Sulphuric acid in the contact process.
solutions


2016
Q1
A, B, C and D summarize the properties of sulphuric acid dependmg
whether it is dilute or concentrated.
A = Typical acid property
B = Non volatile acid
C = Oxidizing agent
D = Dehydrating agent
solutions
A = Typical acid property
B = Non volatile acid
C = Oxidizing agent
D = Dehydrating agent
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment