Class 10 ICSE HCl Board Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter HCl. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
Hydrogen chloride gas is in the laboratory using concentrated
sulphuric acid and sodium chloride. Answer the questions that follow based on this reaction:
(i) Give the balamed chemical for the widl suitable condition(s) if any.
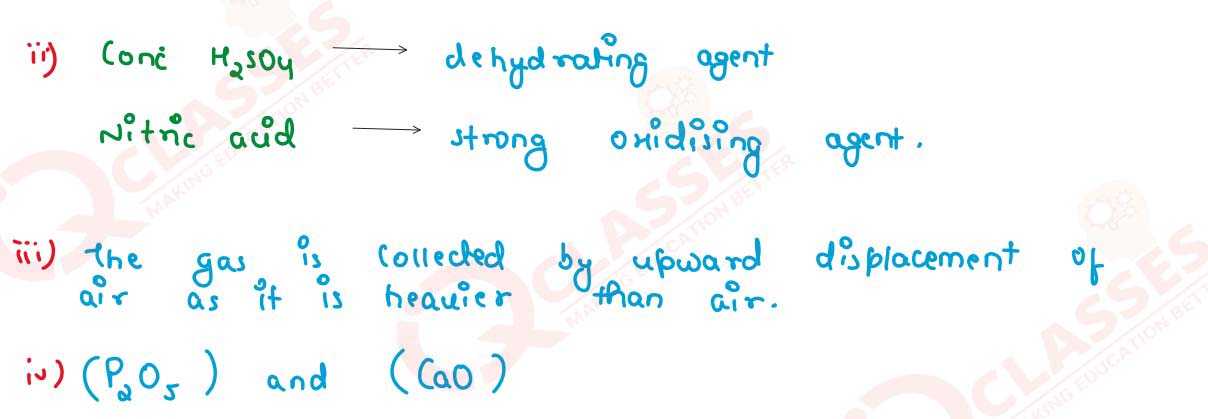
(ii) Why is concentrated sulphuric acid used instead of concentrated nitric acid?
(iii) How is the gas collected?
(iv) Name the drying agent not used for drying the gas.
solutions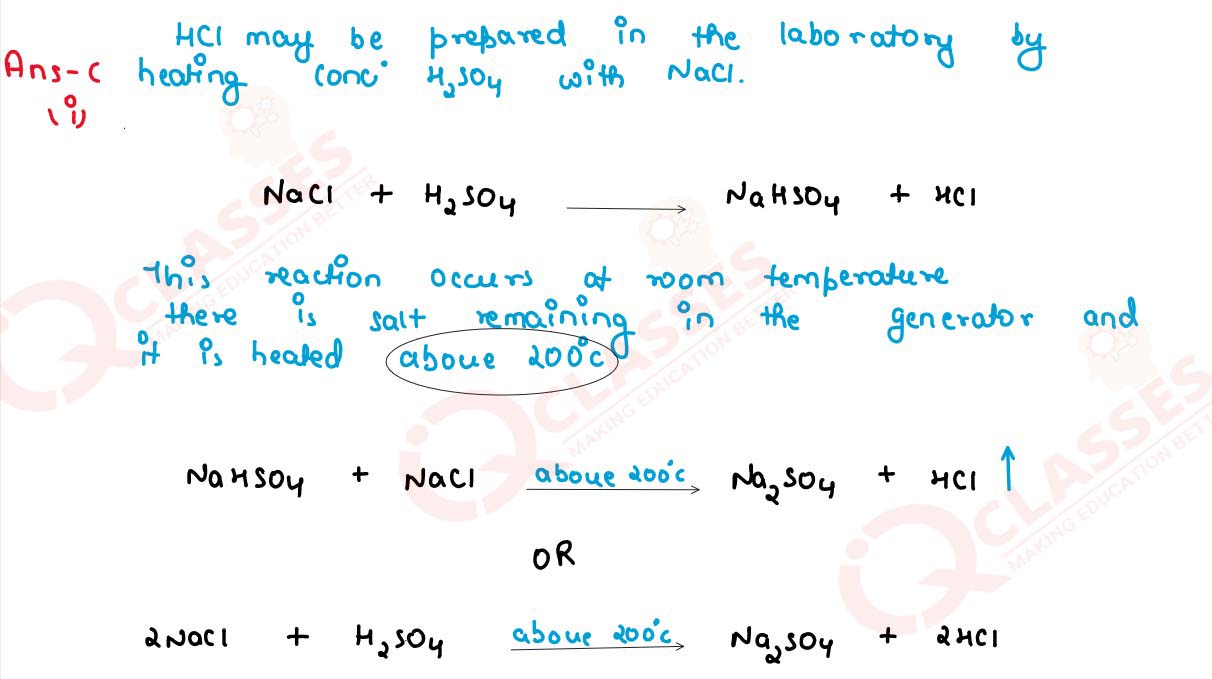
(i) Give the balamed chemical for the widl suitable condition(s) if any.
(ii) Why is concentrated sulphuric acid used instead of concentrated nitric acid?
(iii) How is the gas collected?
(iv) Name the drying agent not used for drying the gas.
solutions


Q2
The Indicator which doesnot change colour on passage of hydrogen chloride is?
the acid which cannot act as an oxidizing agent _______. conc. H2SO4 HNO3 HCl)
solutions
the acid which cannot act as an oxidizing agent _______. conc. H2SO4 HNO3 HCl)
solutions

2019
Q1
The drying agent used to dry HCl gas is;
A. Conc. H2S04
B. ZnO
3. Al2O3
4. CaO
solutions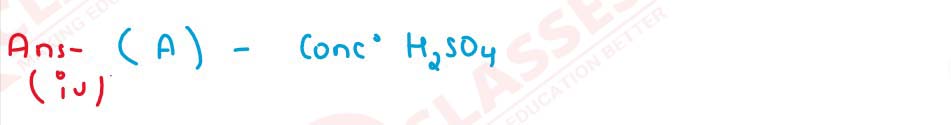
A. Conc. H2S04
B. ZnO
3. Al2O3
4. CaO
solutions
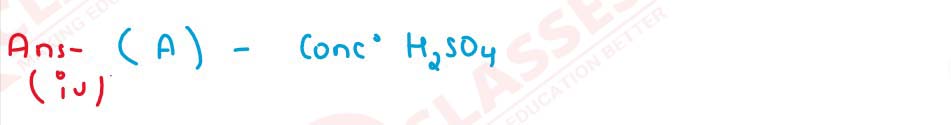
2018
Q1
b) (i) Name the acid used for the preparation of hydrogen chloride gas in the
laboratory. Why is this particular acid preferred to other acids?
(ii) Write the balanced chemical equation for the laboratory preparation of hydrogen chloride gas.
c) For the preparation of hydrochloric acid in the laboratory:
(i) Why is direct absorption of hydrogen chloride gas in water not feasible?
solutions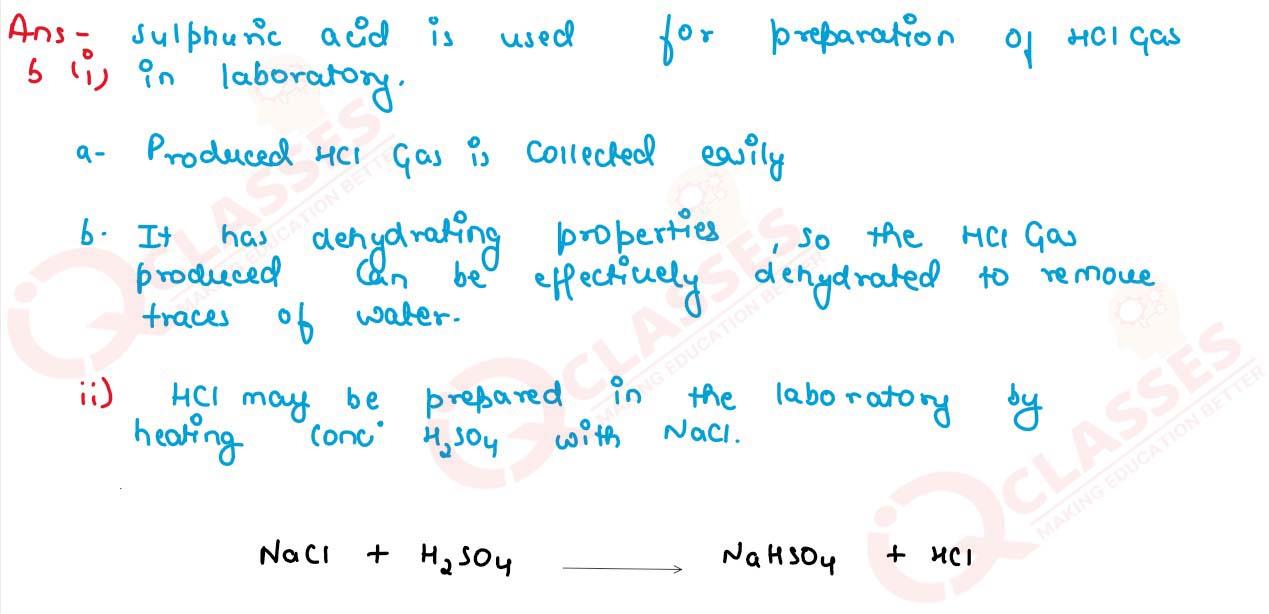

(ii) Write the balanced chemical equation for the laboratory preparation of hydrogen chloride gas.
c) For the preparation of hydrochloric acid in the laboratory:
(i) Why is direct absorption of hydrogen chloride gas in water not feasible?
solutions


2017
Q1
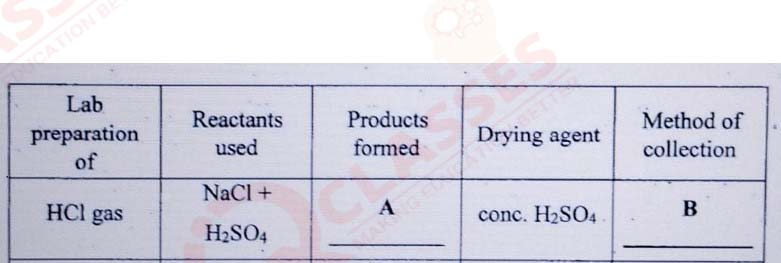
Certain blank spaces are left in the following table and these are labelled as A,
B. Identify each of them.
solutions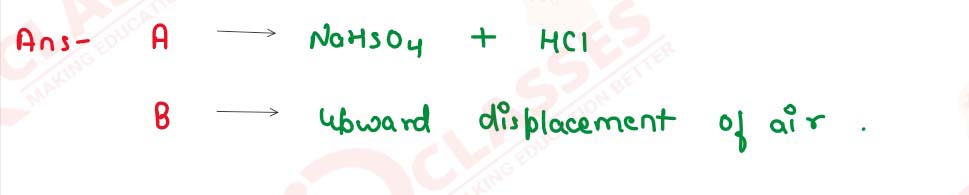

solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment