Class 12 Chemistry CBSE Coordination Compounds Board Questions
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter Coordination Compounds. These important notes,board questions and predicted questions are based on CBSE board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
2020
Q1
The crystal field splitting energy for octahedral (△o) and tetrahedral
(△t) complex is related as
(a) △t = (2/9) △o
(b) △t = (5/9) △o
(c) △t = (4/9) △o
(d) △t = 2 △o
solutions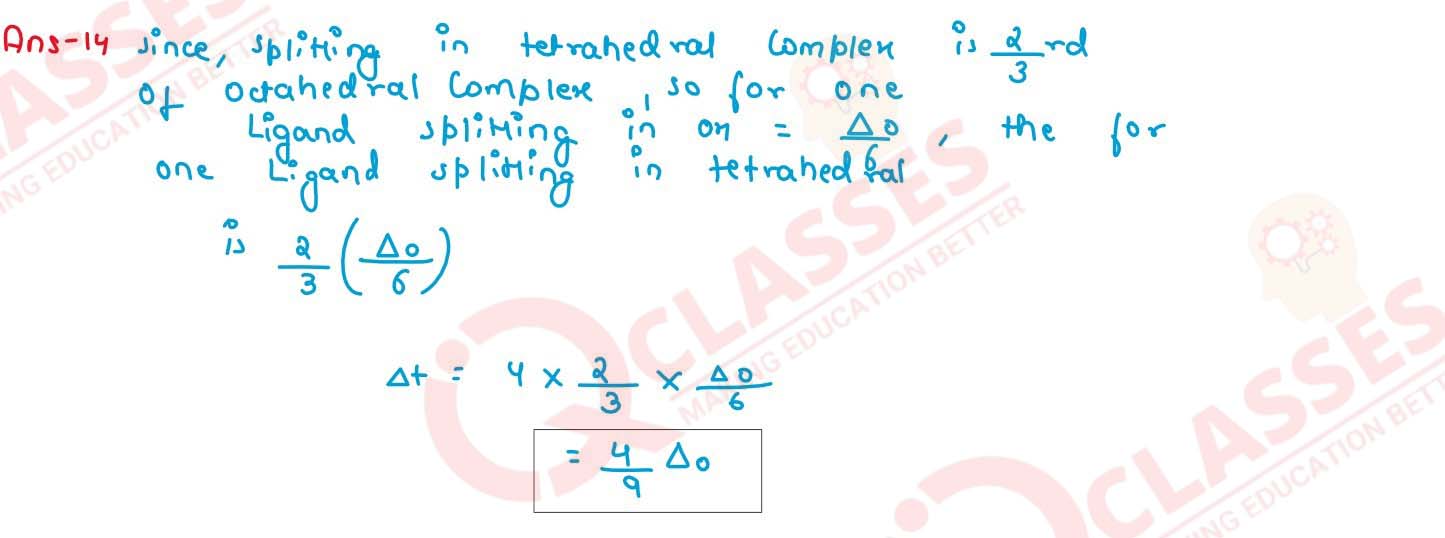
(a) △t = (2/9) △o
(b) △t = (5/9) △o
(c) △t = (4/9) △o
(d) △t = 2 △o
solutions

Q2
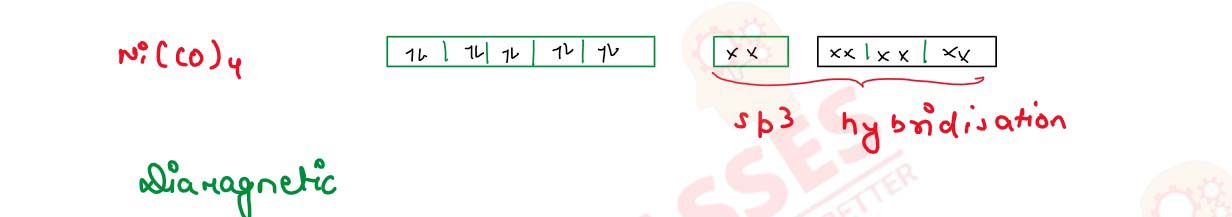
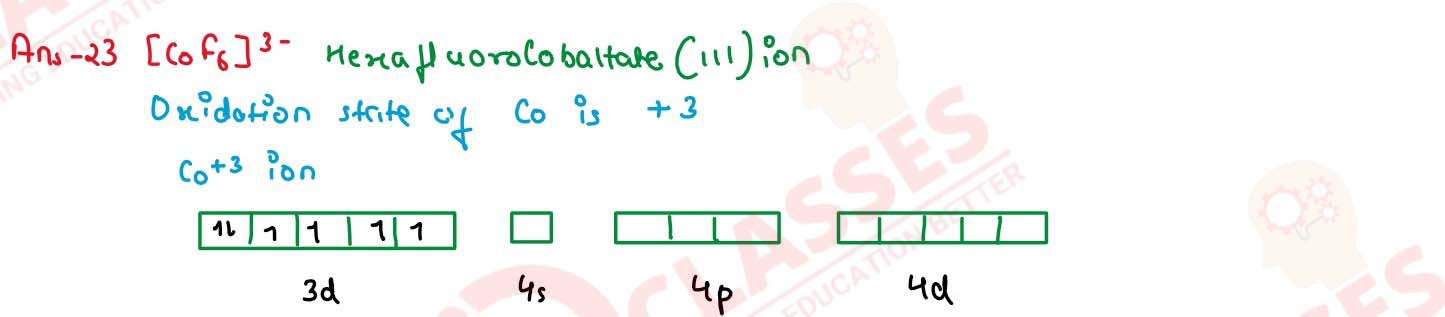
Write IUPAC name and hybridization of the following complexes:
(i) [NI(Co)4]
(ii) [CoF6]3-
(Atomic number Ni = 28 , Co = 27)
solutions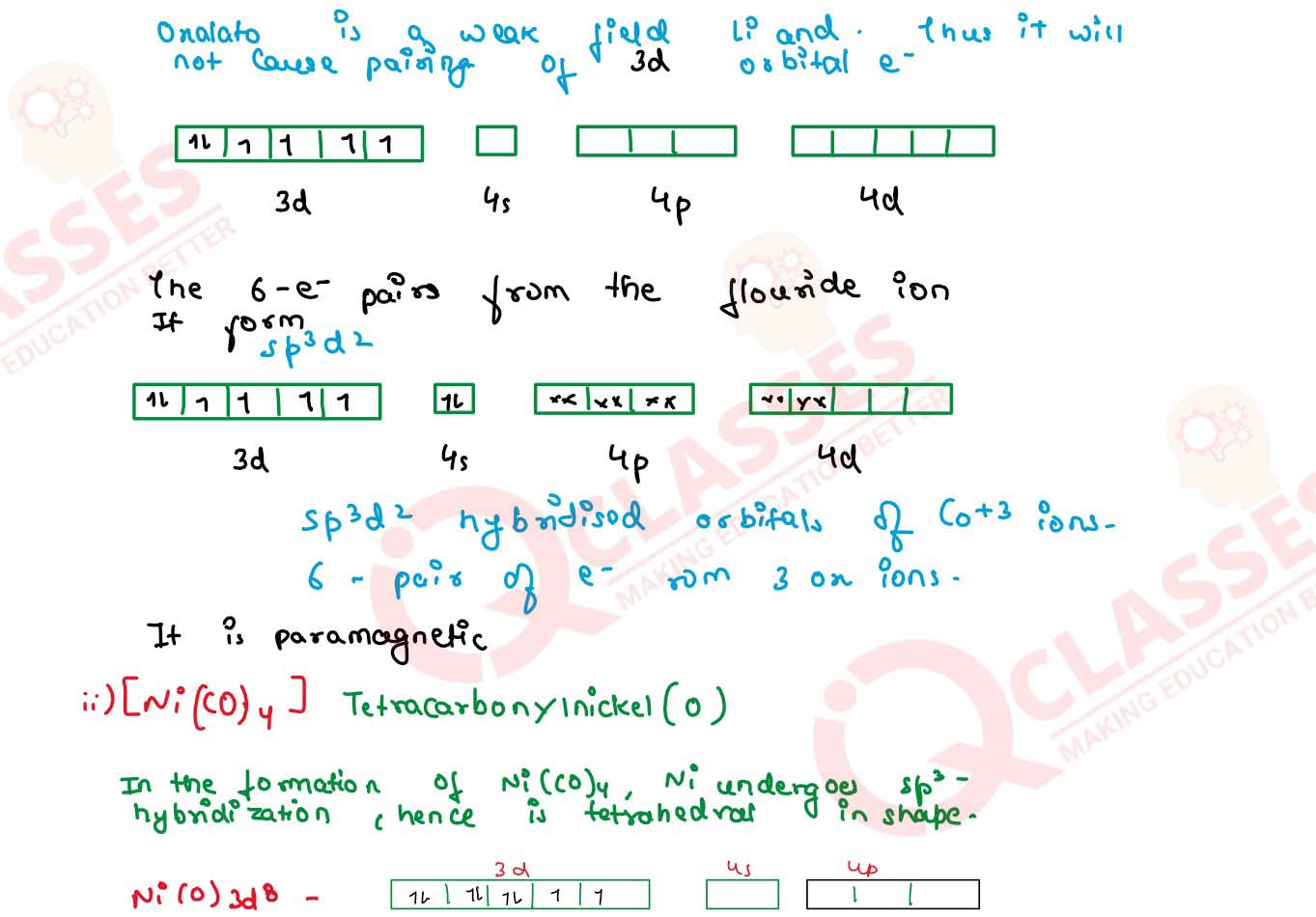
(i) [NI(Co)4]
(ii) [CoF6]3-
(Atomic number Ni = 28 , Co = 27)
solutions



2019
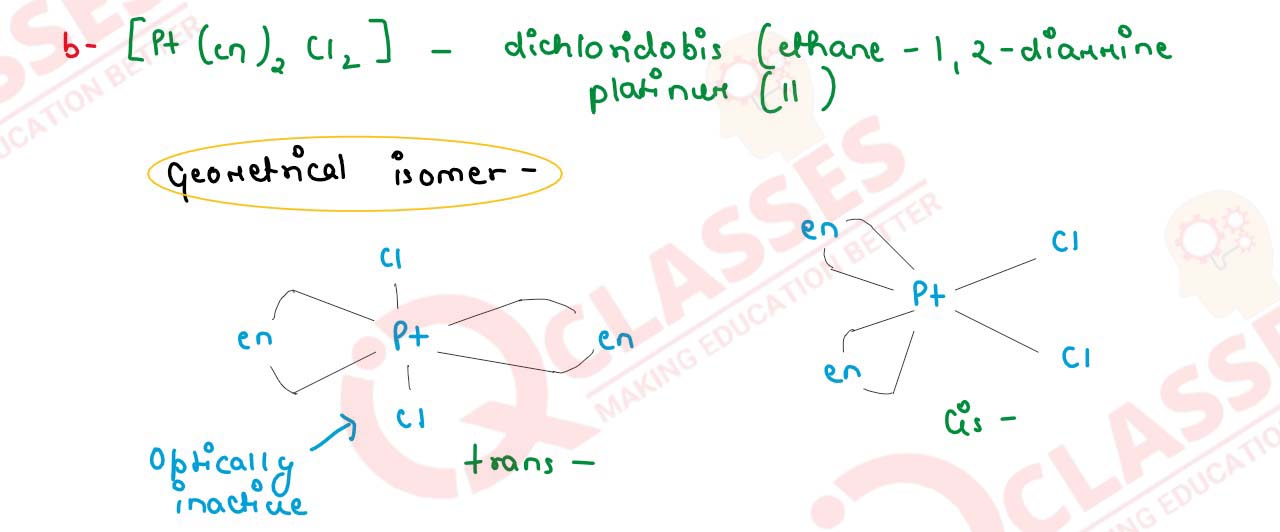
Q3
Write IUPAC name of the complex [Pt(en)2Cl2]. Draw structures of geometrical
isomerism of this complex.
solutions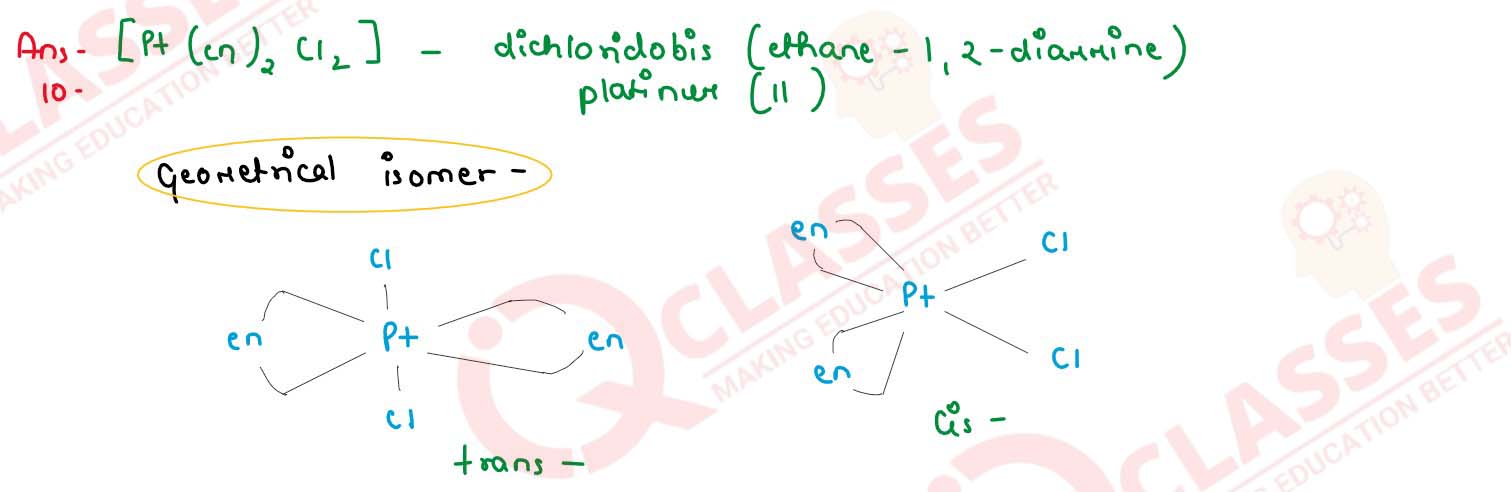
solutions

OR
Q4
Out of [CoF6]3- and [Co(en)3]3+ , which one complex is :
(i) Paramagnetic
(ii) more complex
(iii) inner orbital complex
(iv) high spin complex
(At. no. Co =27)
solutions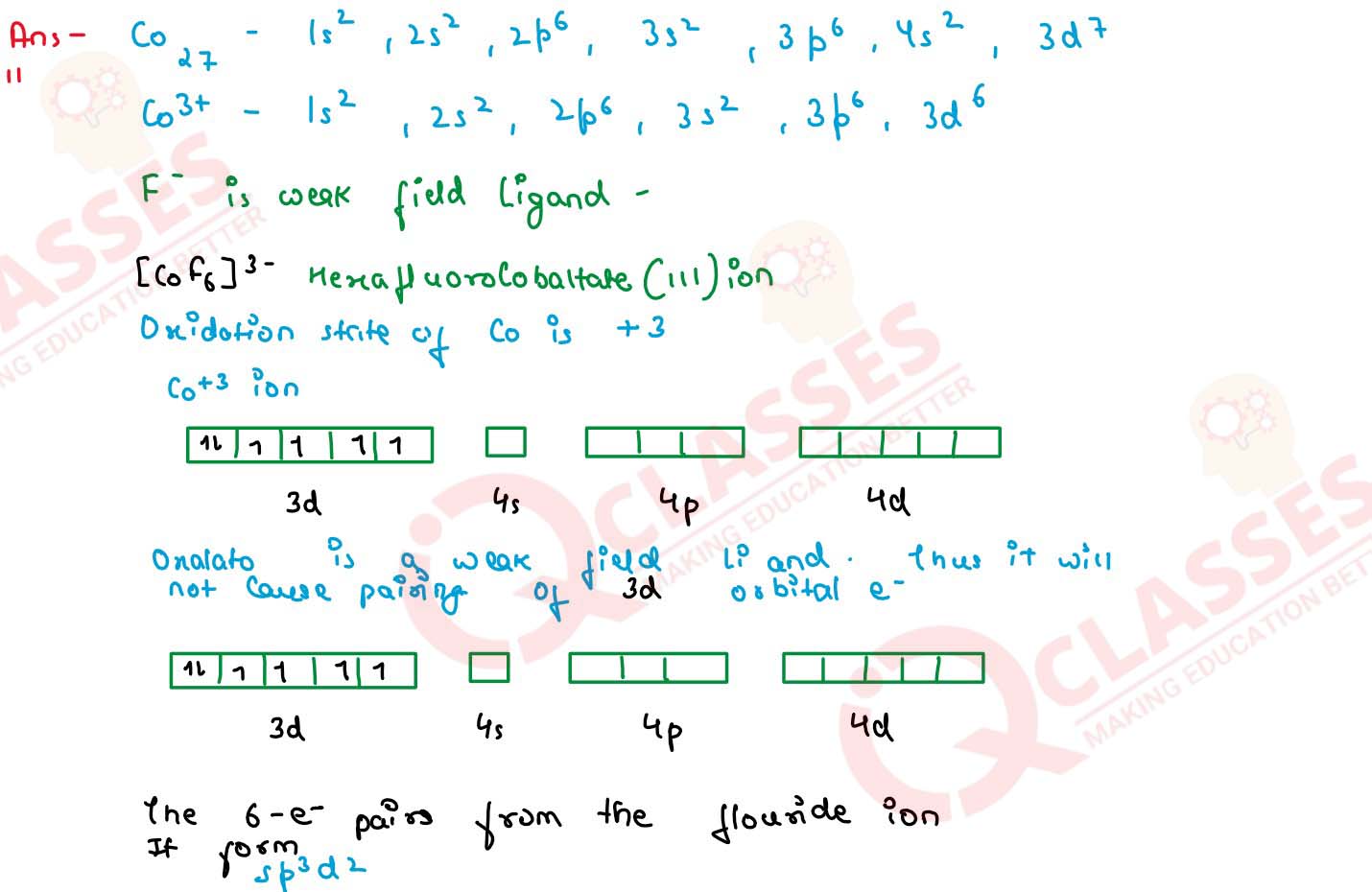
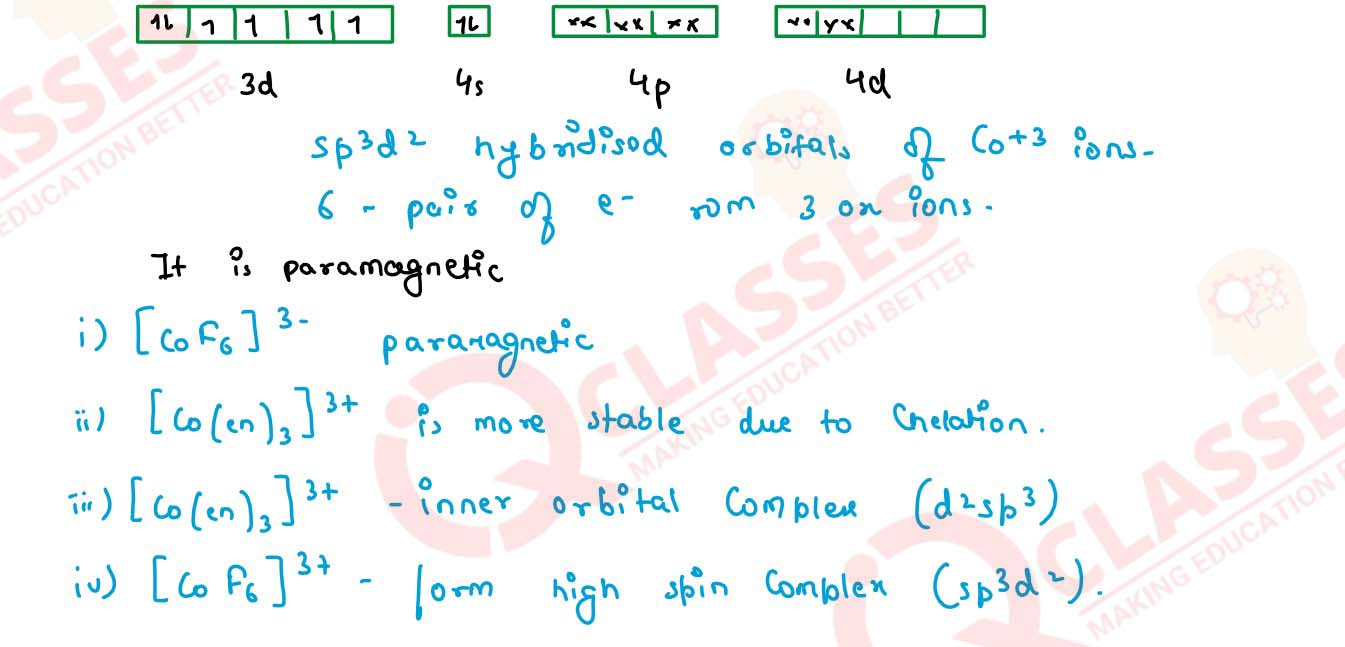
(i) Paramagnetic
(ii) more complex
(iii) inner orbital complex
(iv) high spin complex
(At. no. Co =27)
solutions


2017
Q5
Using of IUPAC norms write the formula for the following :
(a) sodium dicyanidoaurate (I)
(b) Tetraamminechloridonitrito-N-plantinum (IV) sulphate
solutions
(a) sodium dicyanidoaurate (I)
(b) Tetraamminechloridonitrito-N-plantinum (IV) sulphate
solutions


Q6
(a) What type of isomerism is shown by complex
[Co(NH3)5(SCN)]2+
(b) Why is [NiCl4]2- paramagnetic while [Ni(CN)4]2- is diamagnetic ?
(c) The are low spin tetrahedral complexes rarely observed ?
solutions
(b) Why is [NiCl4]2- paramagnetic while [Ni(CN)4]2- is diamagnetic ?
(c) The are low spin tetrahedral complexes rarely observed ?
solutions

2016
Q7
(a) For complex [Fe(H2O)6S]3+, write the hybridization , magnetic
character and spin of the complex. (At. number : Fe = 26)
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2s]2+ which is optically inactive.
solutions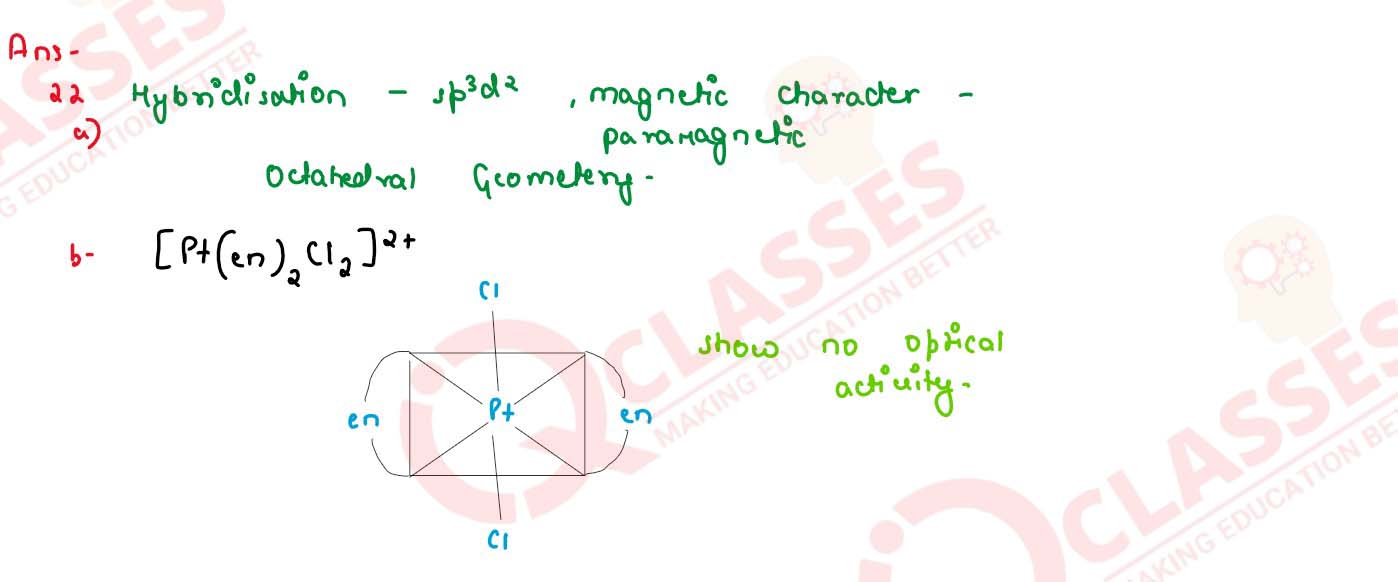
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2s]2+ which is optically inactive.
solutions



Add a comment