Class 12 Chemistry CBSE Electrochemistry Mostlikely QuestionBank
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter Electrochemistry. These important notes,board questions and predicted questions are based on CBSE board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
class 12 CBSE Electrochemistry Mostlikely-QuestionBank
Electrochemistry Mostlikely-QuestionBank
Q1
What is cell constant ?
solutions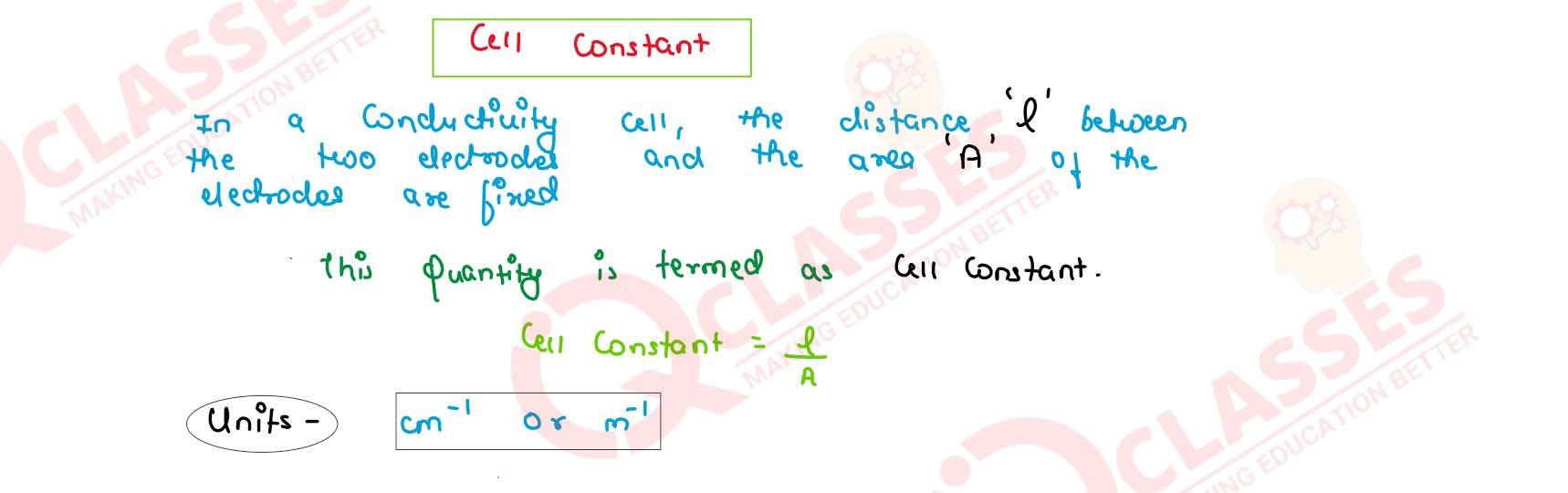
solutions

Q2
(a) What type of a battery is the lead storage battery? Write the anode and cathode reaction in the
overall reaction occurring in a lead storage battery when current is drawn from it
(b) In the button cell , widely used in watches the following reaction takes place :
Zn(s) + Ag2O (s) + H2O (I) → Zn2+(aq) + 2Ag(s) + 2 OH-(aq)
Determine Eo and △Go for the reaction
(Given : EoAg+/Ag = + 0.80 V, EoZn2+ /Zn = -0.76 V)
solutions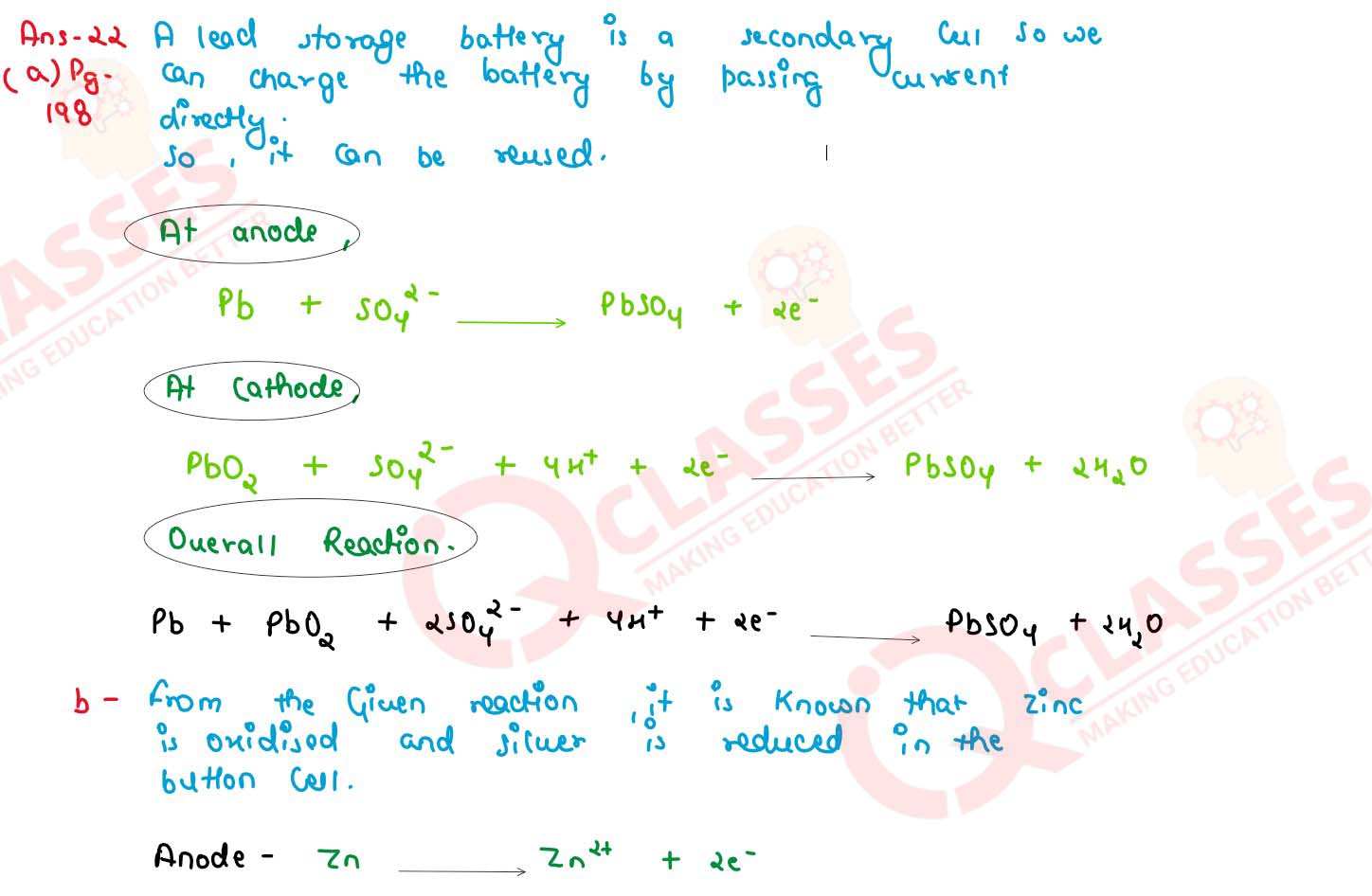
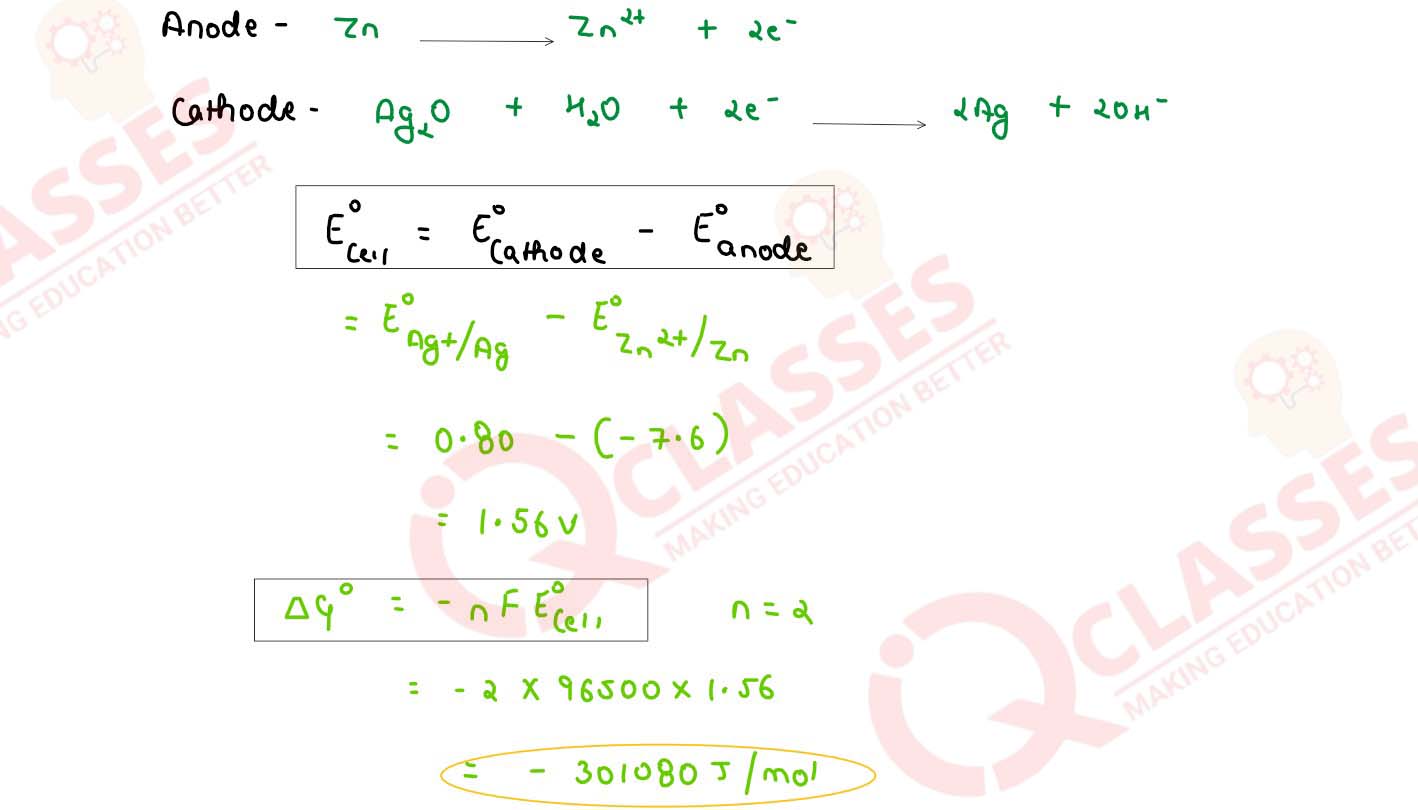
(b) In the button cell , widely used in watches the following reaction takes place :
Zn(s) + Ag2O (s) + H2O (I) → Zn2+(aq) + 2Ag(s) + 2 OH-(aq)
Determine Eo and △Go for the reaction
(Given : EoAg+/Ag = + 0.80 V, EoZn2+ /Zn = -0.76 V)
solutions


Q3
Define fuel cell and write its 2 advantages
solutions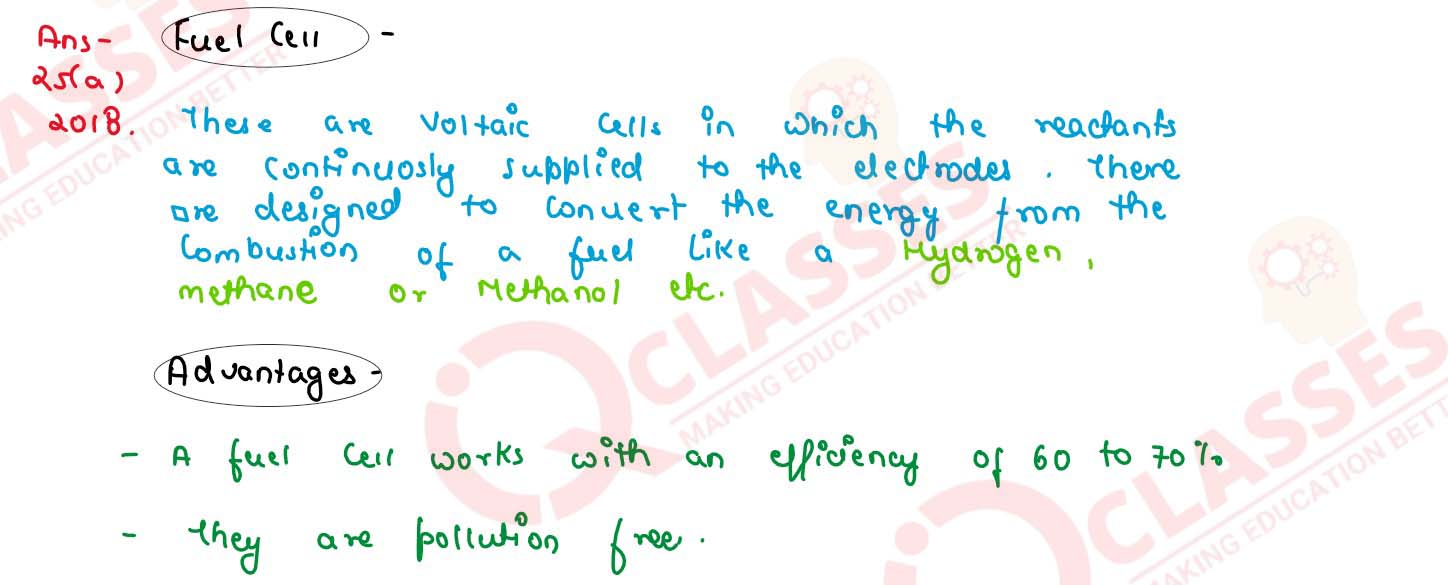
solutions

Q4
Define the term molar conductivity for the solution of an electrolyte. Comment on their variation
with temperature.
solutions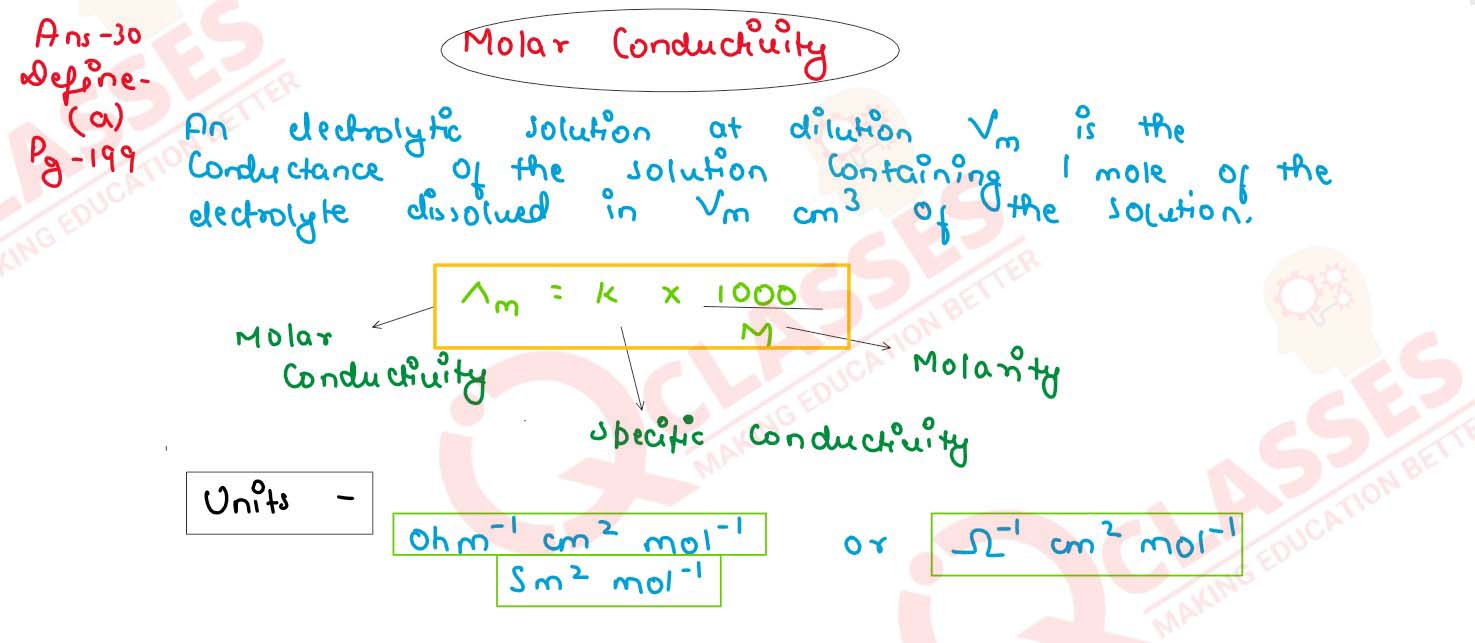
solutions

Q5
The measured resistance of a conductance cell was 100 ohms. Calculate (i) the specific conductance
and (ii) molar conductance of the solution.
(KCl = 74.5 g mol-1 and cell constant = 1.25 cm-1)
solutions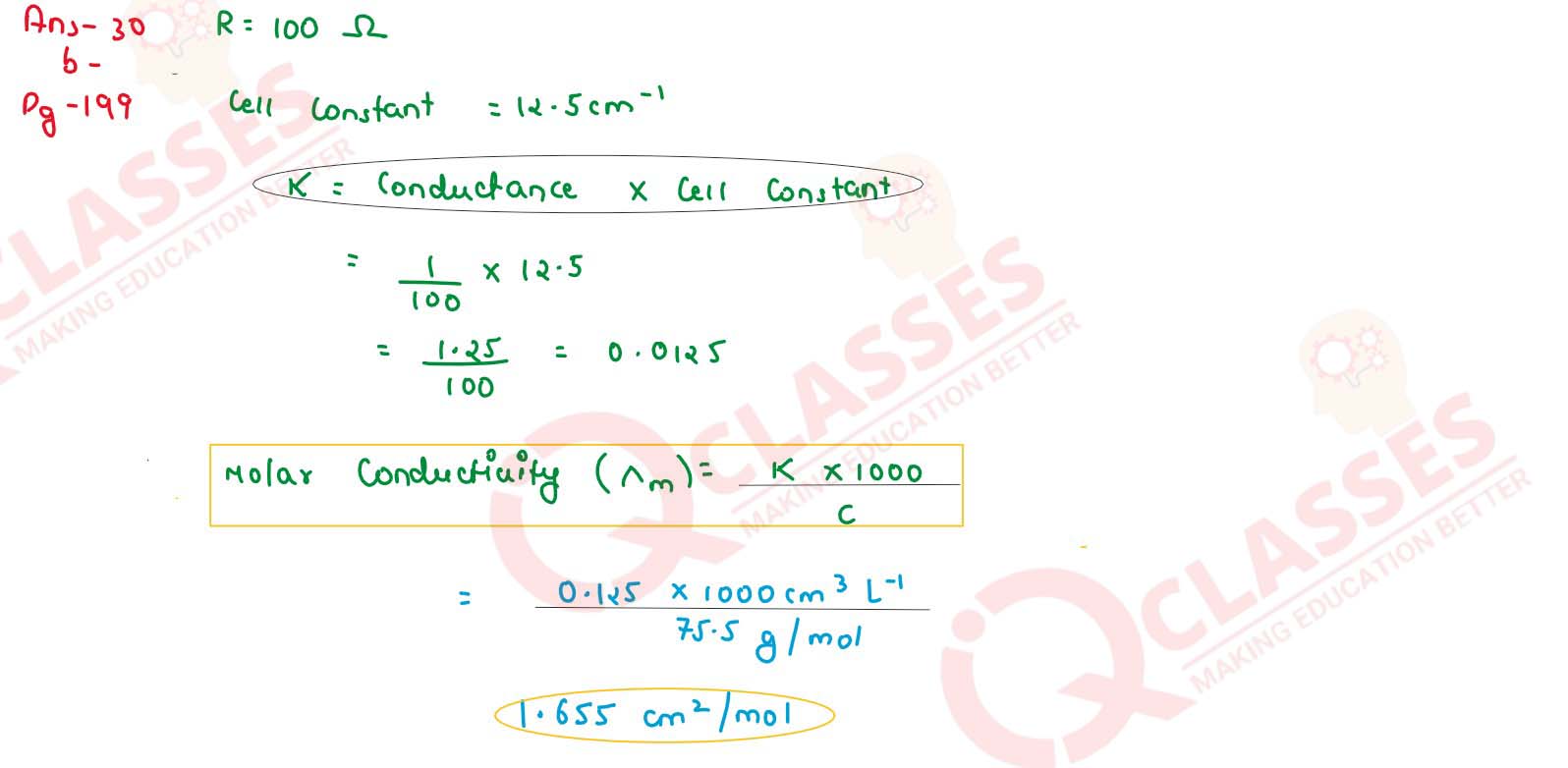
(KCl = 74.5 g mol-1 and cell constant = 1.25 cm-1)
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment