Class 12 Chemistry ISC chemical Kinetics Board Questions
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter Chemical Kinetics. These important notes,board questions and predicted questions are based on ISC board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
2020
Q1
A first order reaction is 50% completed in 30 minutes at 300 K and in 10 minutes at 320 K. Calculate
the activation energy of the reaction. (R = 8.314 JK-1mol-1)
solutions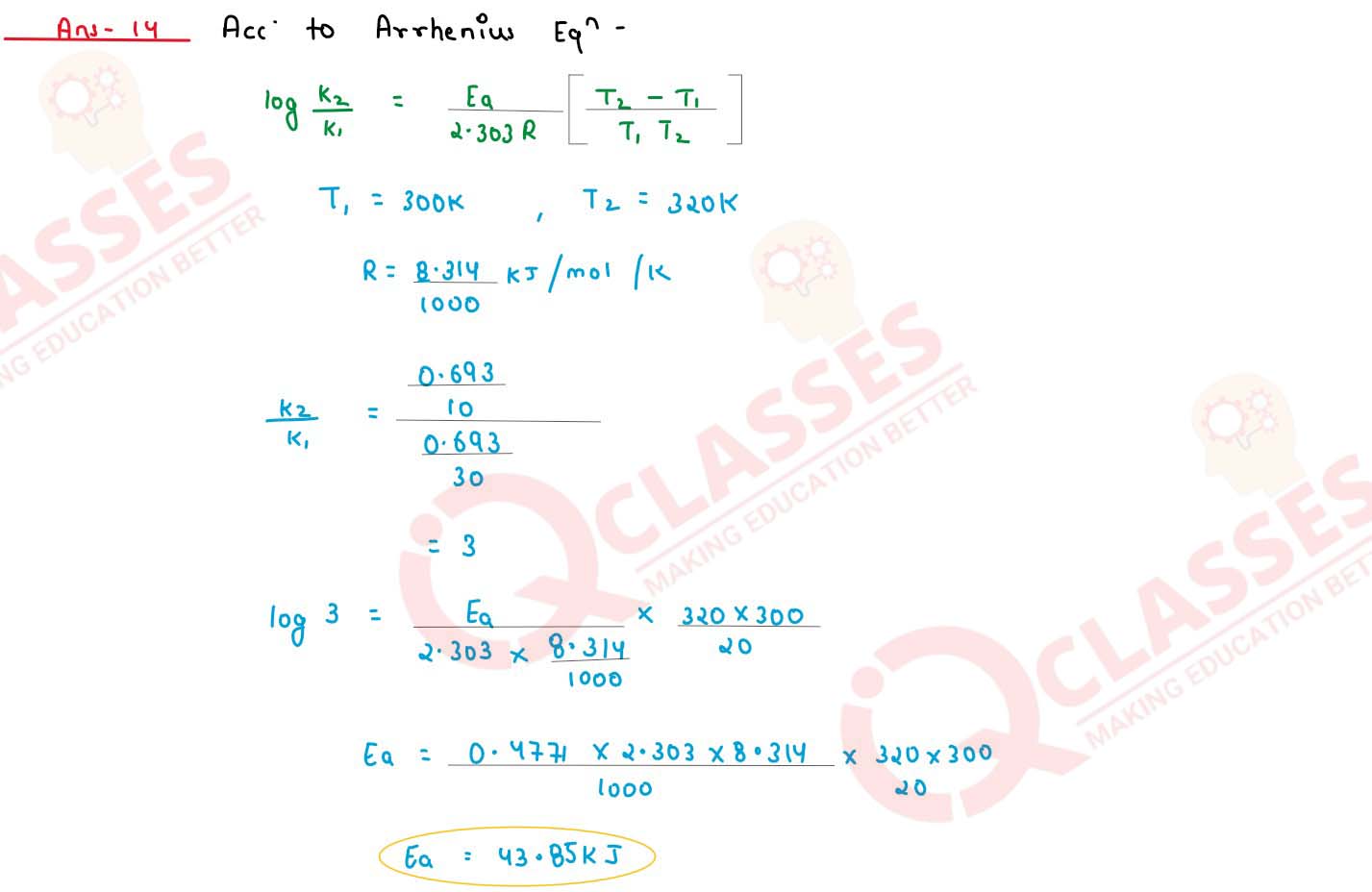


solutions



2019
Q2
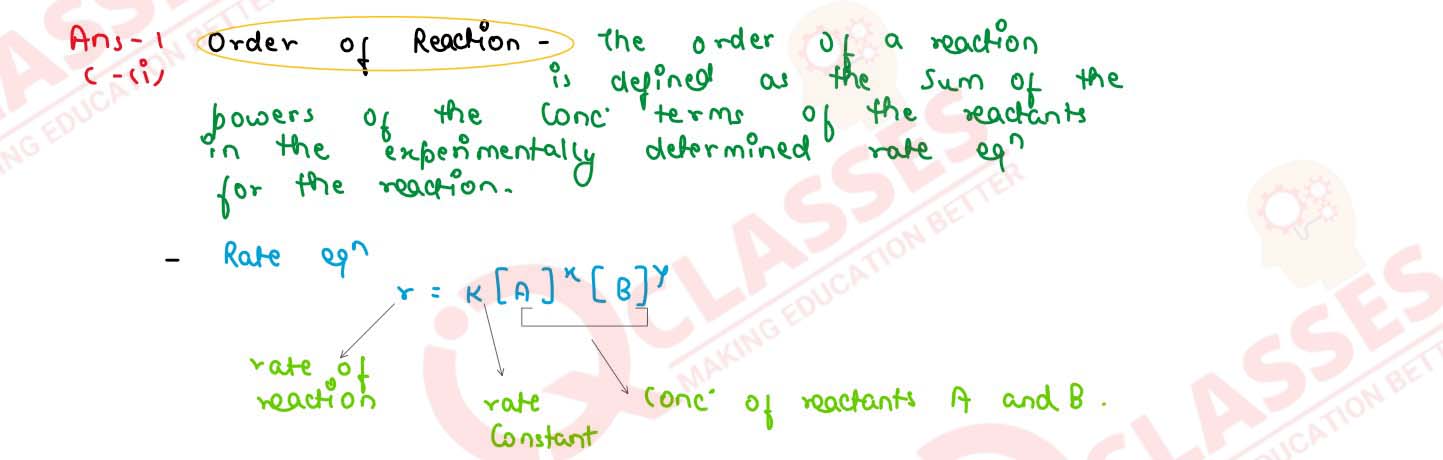
For the reaction the initial rate for different reaction and initial concentration of reactants are
given below:
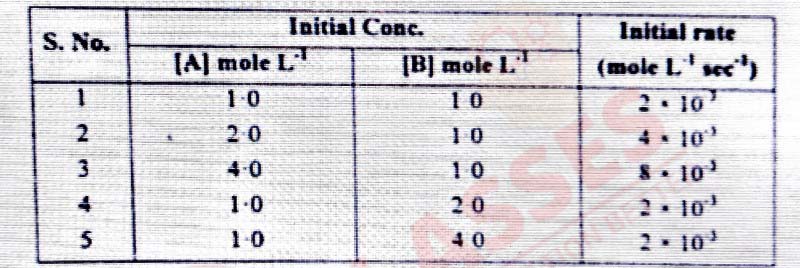
(i) what is the overall order of reaction?
(ii) Write the rate law equation
solutions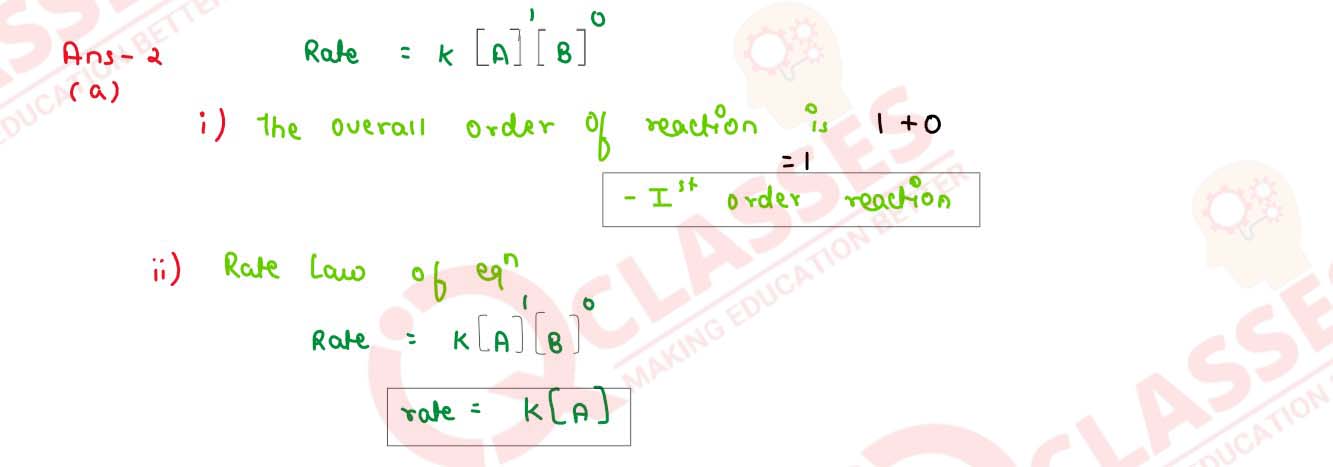




(i) what is the overall order of reaction?
(ii) Write the rate law equation
solutions




OR
Q3
25% of a first order reaction is completed in 30 minutes. Calculate the time taken in minutes for
the reaction to go to 90% completion.
solutions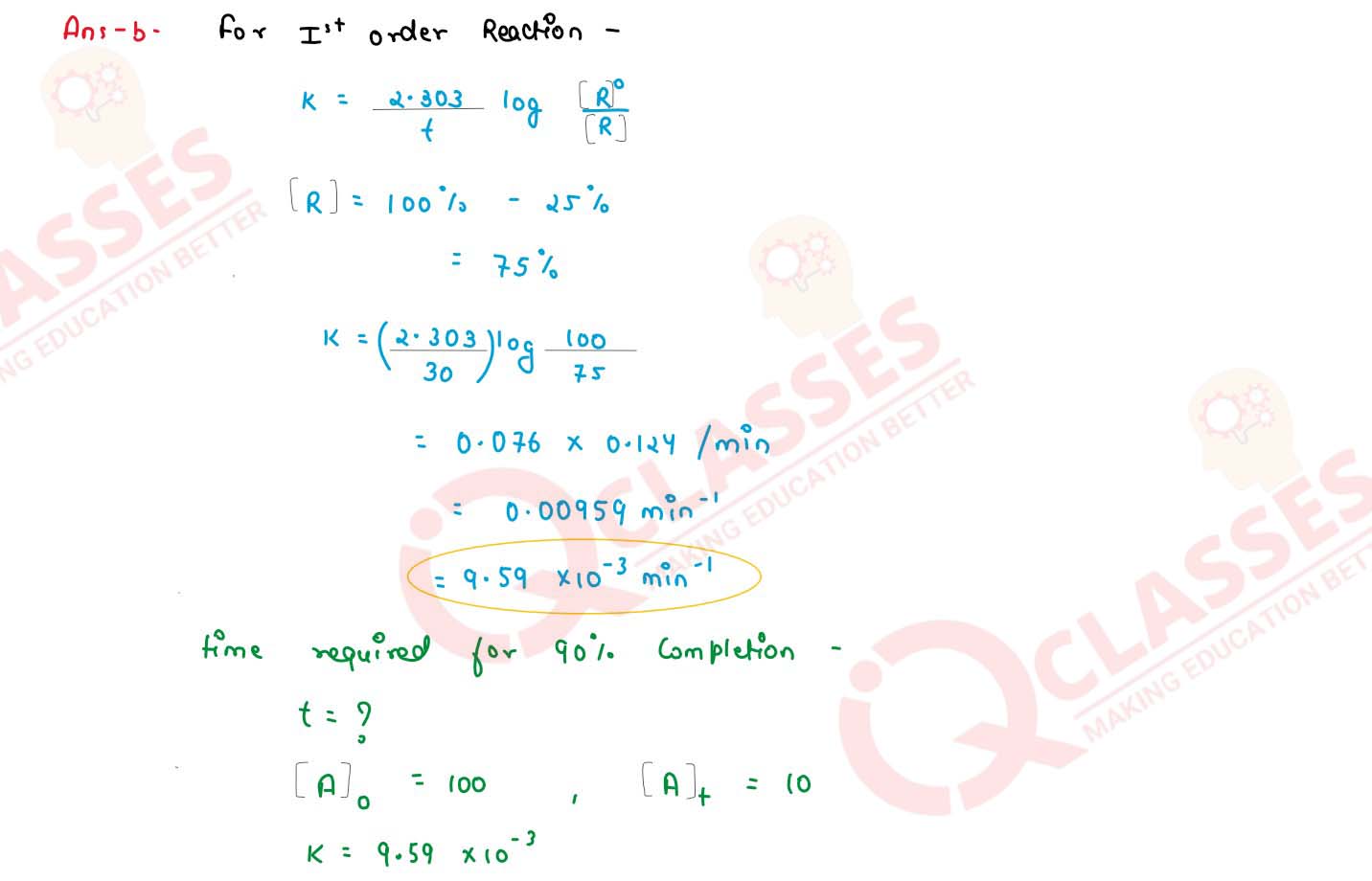

solutions


2018
Q4
(i) Write rate law for the order of reaction is first, second and zero with respect to A,B and C
respectively.
(ii) How many times the rate of reaction will increase if the concentration of A, B and C are doubled in the equation given in (i) above?
solutions

(ii) How many times the rate of reaction will increase if the concentration of A, B and C are doubled in the equation given in (i) above?
solutions


OR
Q5
The rate of reaction becomes 4 times when the temperature changes from 293 K to 313 K. Calculate the
energy of activation (Ea) of the reaction assuming that it doesn't change with
temperature. (R = 8.314 JK-1mol-1)
solutions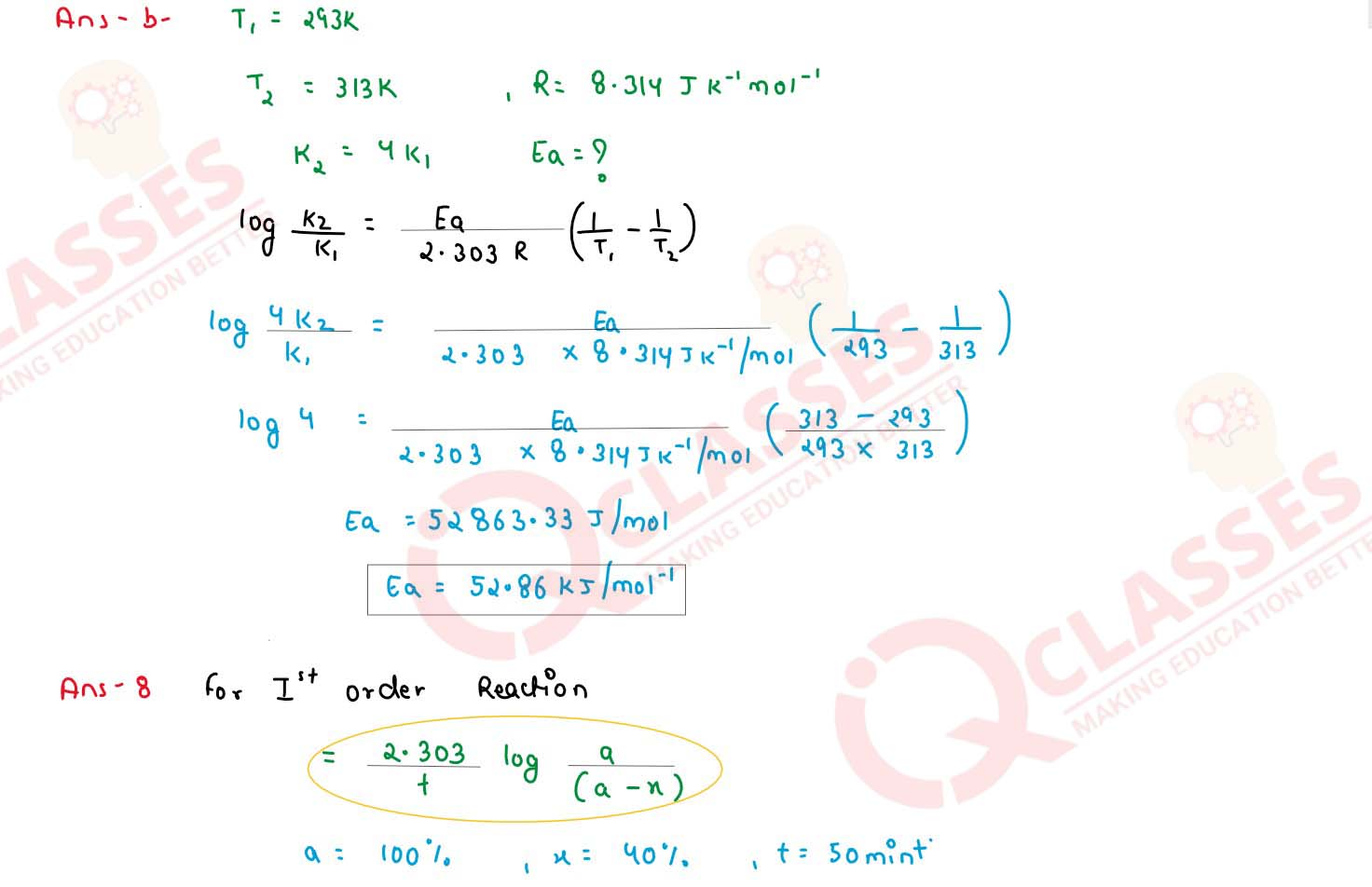
solutions


2017
Q6
Identify the order of reaction from each of the following units of rate constant (k)
(a) mol-1 L sec-1
(b) mol-1 L sec-1
solutions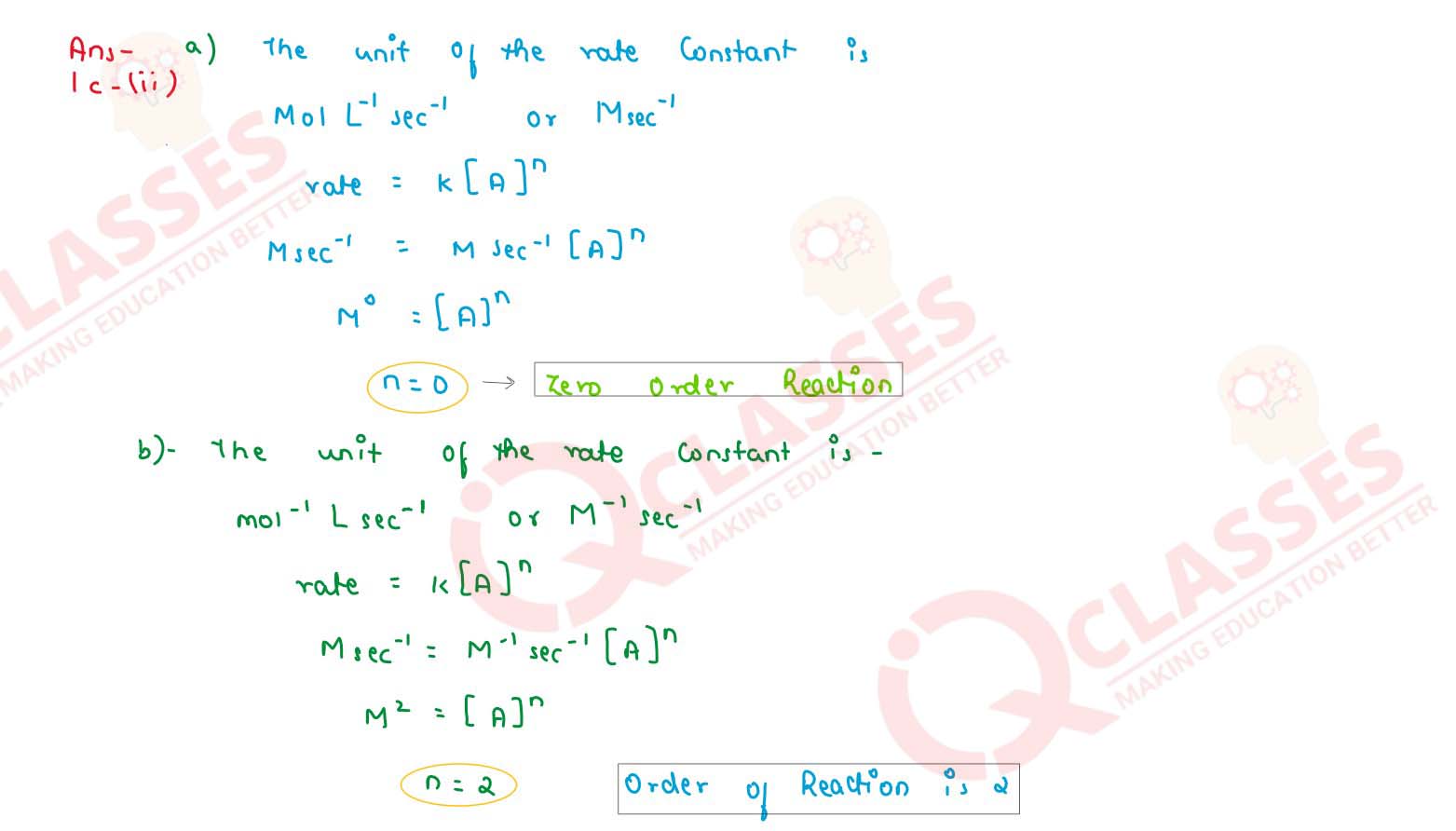
(a) mol-1 L sec-1
(b) mol-1 L sec-1
solutions

Q7
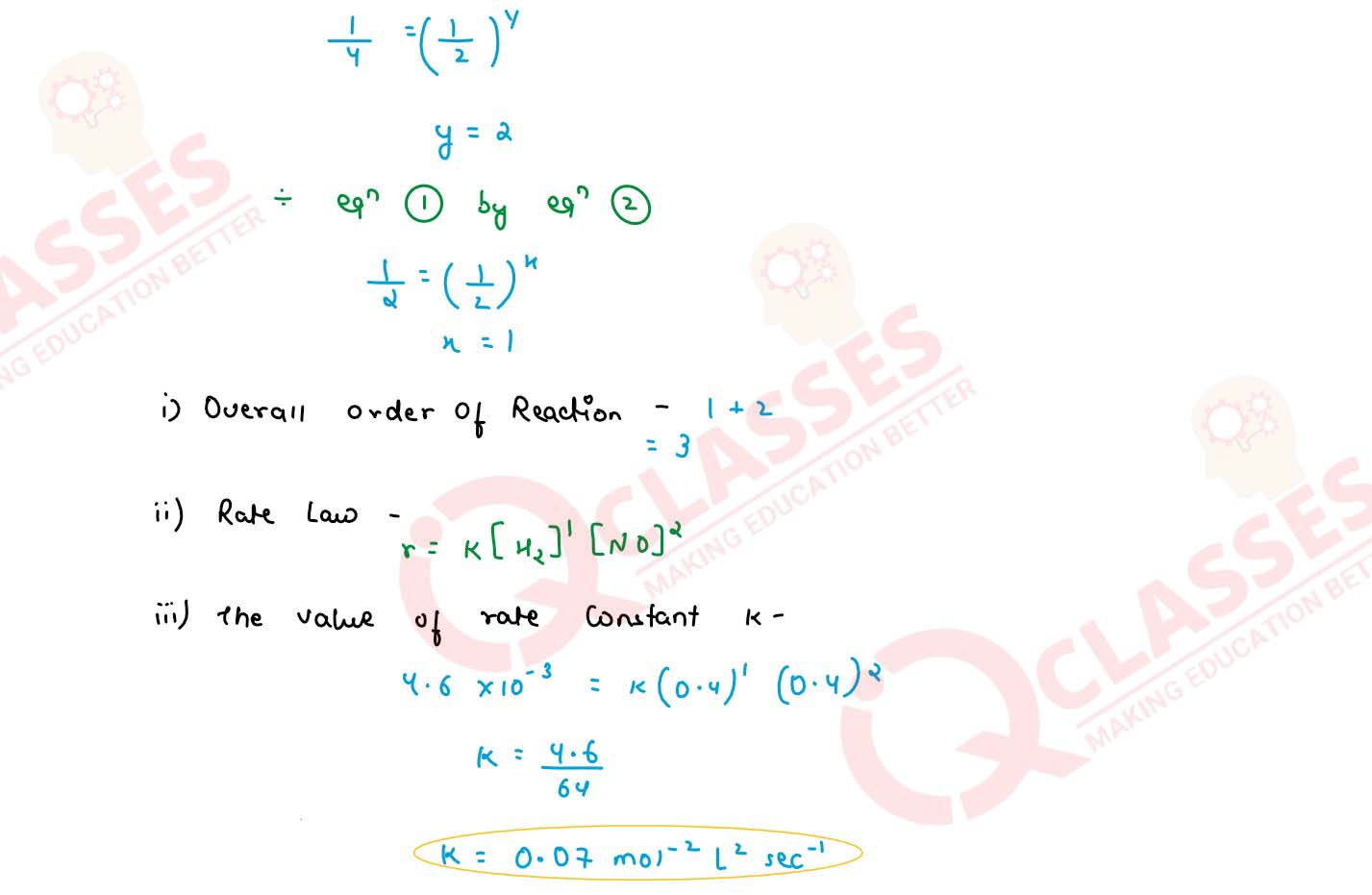
For the reaction 2H2 + 2NO ⇌ 2H2O + N2, the following rate
data was obtained :
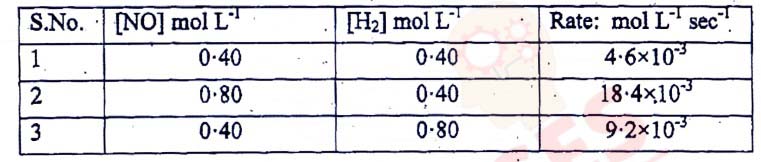
Calculate the following :
(1) The overall order of reaction
(2) The rate law
(3) The value of rate constant(k)
solutions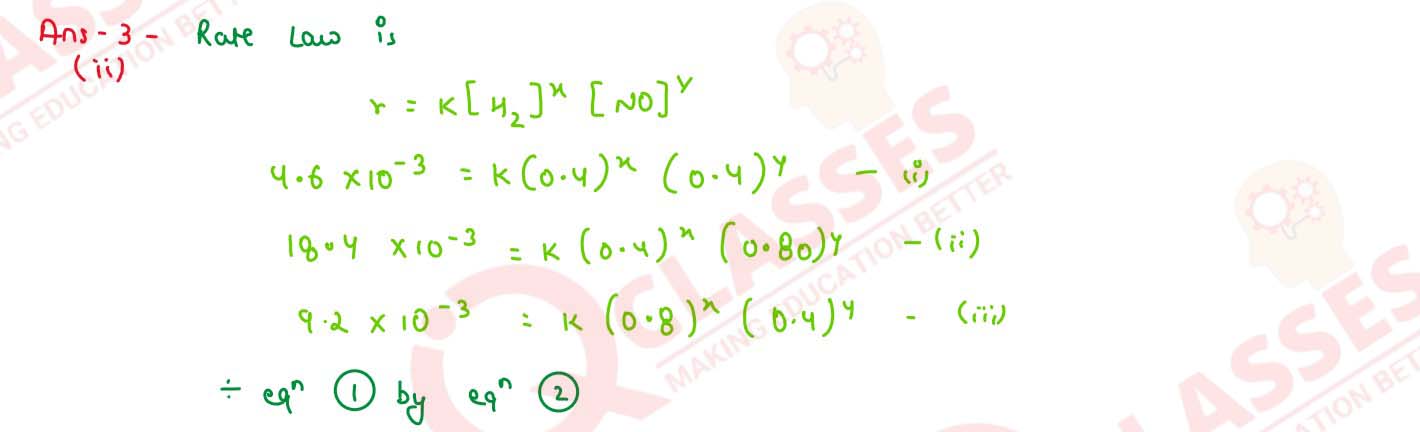

Calculate the following :
(1) The overall order of reaction
(2) The rate law
(3) The value of rate constant(k)
solutions
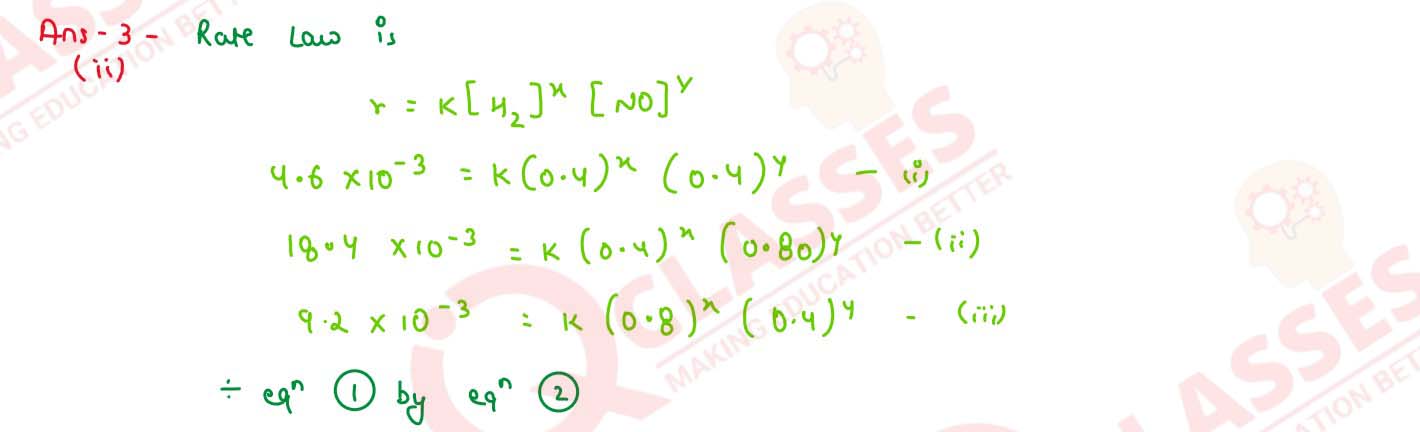

2016
Q8
What is the order of reaction whose rate constant has the same unit as the rate of reaction?
solutions
solutions


Q9
(i) For the reaction : 2NO(g) ⇌ N2(g) + O2(g) ; △H =
-heat
Ke = 2.5 x 102 at 298 K
What will happen to the concentration of N2 if :
(1) temperature in decreased to 273 K
(2) pressure is reduced
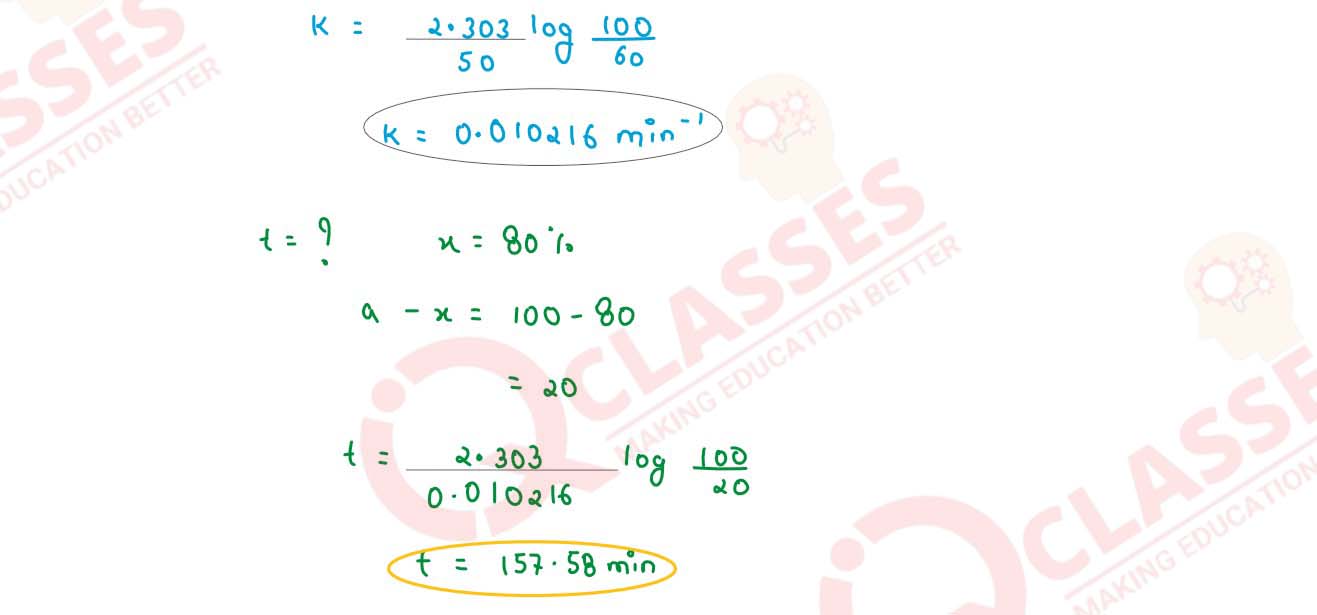
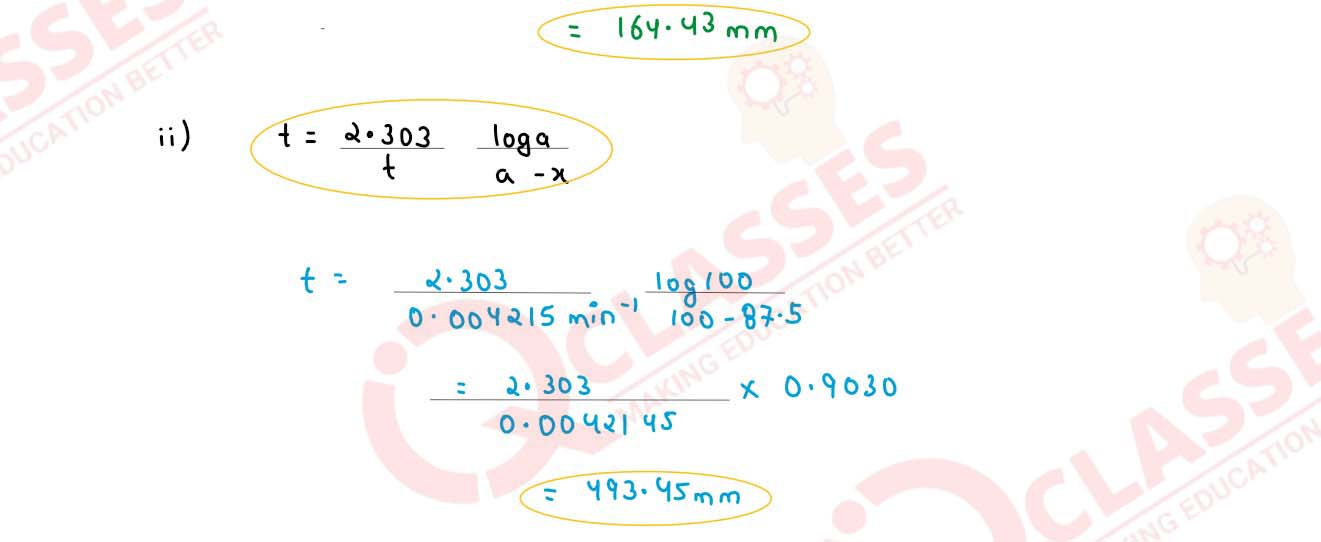
(ii) In a first order reaction 10% of the reactant is consumed in 25 minutes. Calculate:
(1) the half life period of the reaction
(2) the time required for completing 87.5% of the reaction
solutions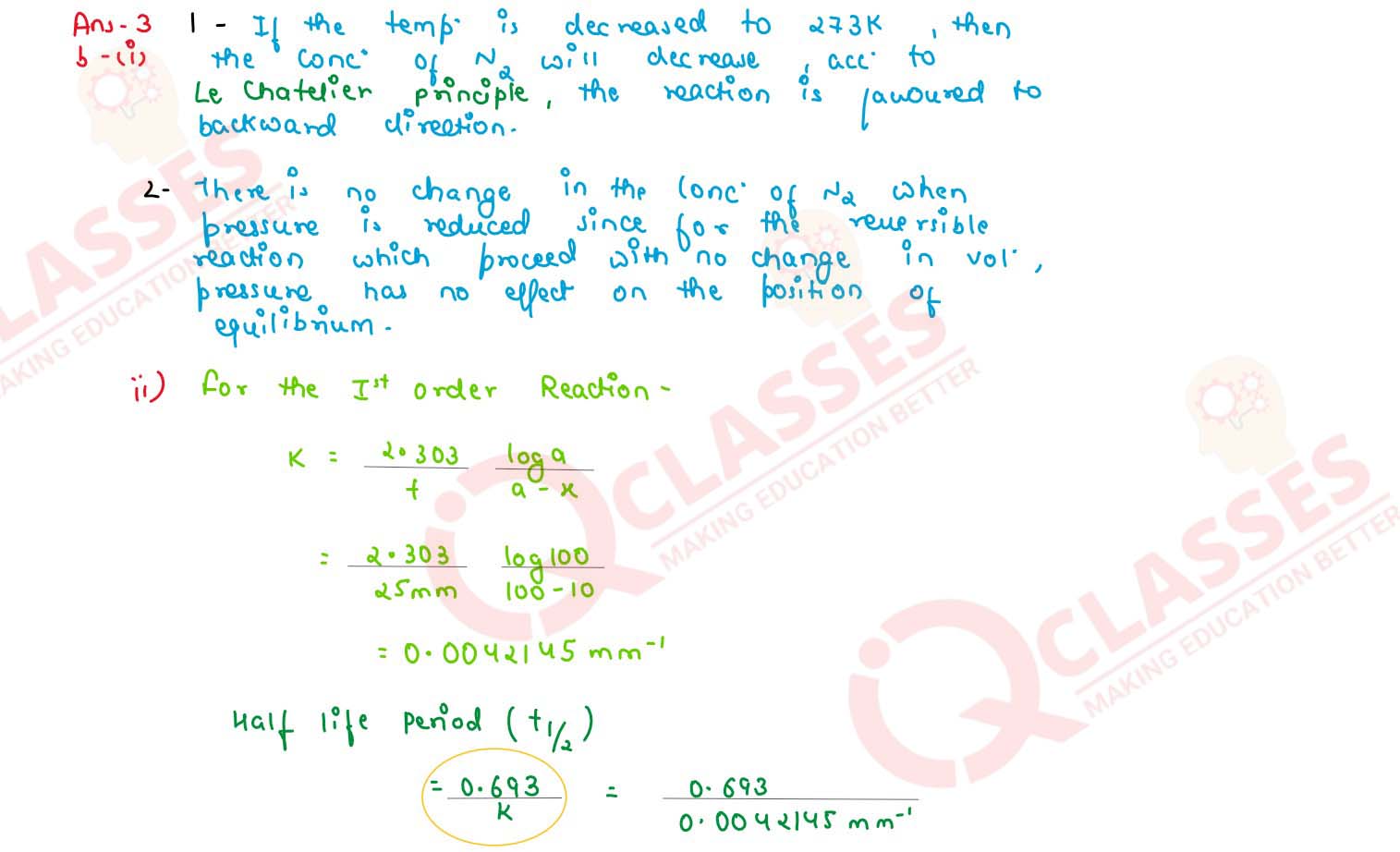
Ke = 2.5 x 102 at 298 K
What will happen to the concentration of N2 if :
(1) temperature in decreased to 273 K
(2) pressure is reduced
(ii) In a first order reaction 10% of the reactant is consumed in 25 minutes. Calculate:
(1) the half life period of the reaction
(2) the time required for completing 87.5% of the reaction
solutions



Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment