Class 12 Chemistry ISC Coordination Compounds Board Questions
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter Coordination Compounds. These important notes,board questions and predicted questions are based on ISC board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
2020
Q1
(a) (i) Write the IUPAC name of the following complexes:
(1) [Cu(NH3)4]SO4
(2) [Co(en)2Cl2]
(3) K3[Al(C2O4)3]
(ii) With reference to the coordination complex ion [Fe(H2O)6]2+ answer the following: (atomic number of Fe = 26)
(1) give the IUPAC name of the complex ion
(ii) what is the oxidation number of the central metal atom
(iii) how many unpaired electrons are there in the complex ion
(iv) state the type of hybridization of the complex ion
(b) (i) Name of the type of isomerism exhibited by the following pairs of compound
(1) [Co(ONO)(NH3)5]2+ and [Co(NO2)(NH3)5]2+
(2) [Cr(H2O)4C3]Cl.2H2O and [Cr(H2O)5Cl]Cl2.2H2O
(3) [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6]
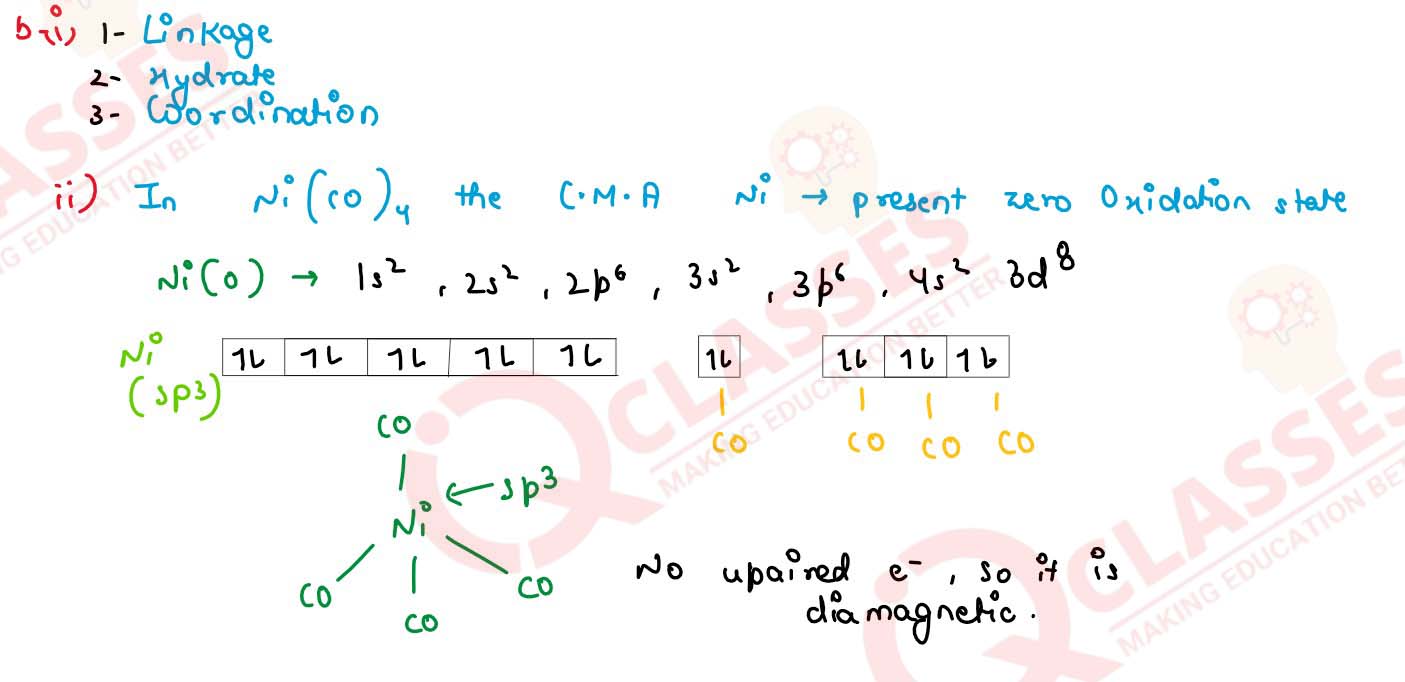
(ii) Using the valence bond approach predict the shape, hybridisation and magnetic behaviour of [Ni(Co4)] (at no of Ni =28)
solutions
(1) [Cu(NH3)4]SO4
(2) [Co(en)2Cl2]
(3) K3[Al(C2O4)3]
(ii) With reference to the coordination complex ion [Fe(H2O)6]2+ answer the following: (atomic number of Fe = 26)
(1) give the IUPAC name of the complex ion
(ii) what is the oxidation number of the central metal atom
(iii) how many unpaired electrons are there in the complex ion
(iv) state the type of hybridization of the complex ion
(b) (i) Name of the type of isomerism exhibited by the following pairs of compound
(1) [Co(ONO)(NH3)5]2+ and [Co(NO2)(NH3)5]2+
(2) [Cr(H2O)4C3]Cl.2H2O and [Cr(H2O)5Cl]Cl2.2H2O
(3) [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6]
(ii) Using the valence bond approach predict the shape, hybridisation and magnetic behaviour of [Ni(Co4)] (at no of Ni =28)
solutions


2019
Q2
(i) Write the IUPAC name of the following :
(1) K3[Fe(Cr2O4)4]
(2) [Co (NH3)5Cl]SO4
(ii) [Fe(CN)6]4- is a coordination complex ion.
(1) Calculate the oxidation number of iron in the complex.
(2) Is the complex ion diamagnetic or paramagnetic?
(3) What is the hybridization state of the central metal atom
(4) write the IUPAC name of the complex ion
solutions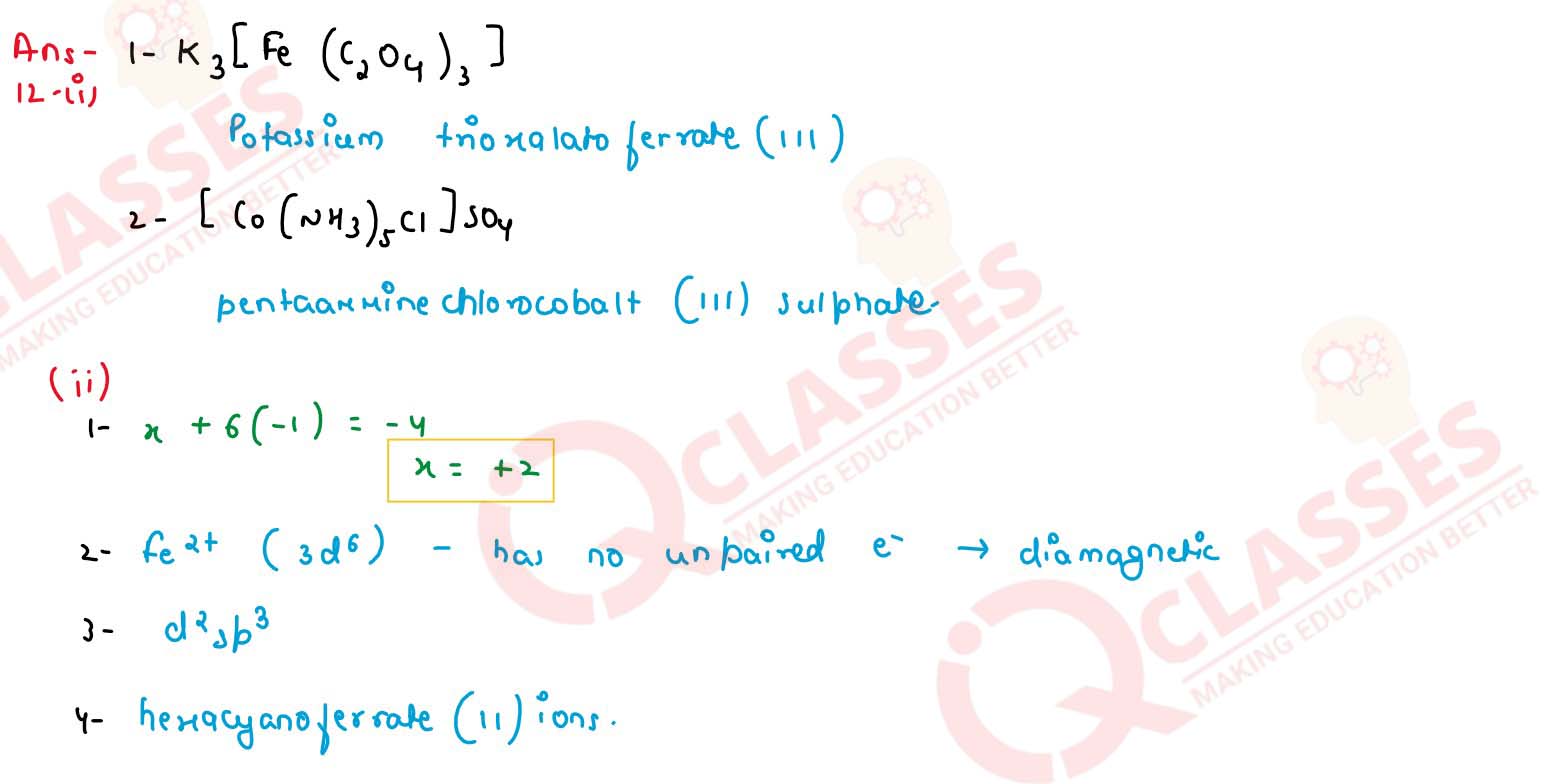
(1) K3[Fe(Cr2O4)4]
(2) [Co (NH3)5Cl]SO4
(ii) [Fe(CN)6]4- is a coordination complex ion.
(1) Calculate the oxidation number of iron in the complex.
(2) Is the complex ion diamagnetic or paramagnetic?
(3) What is the hybridization state of the central metal atom
(4) write the IUPAC name of the complex ion
solutions

2018
Q3
(a) For the complex ion [Fe(CN)6]3- state:
(i) the type of hybridisation.
(ii) the magnetic behavoiur.
(iii) the oxidation number of the central metal atom.
(b) Write the IUPAC name of [Co(en)2Cl2]+ ion and draw geometrical isomerism.
solutions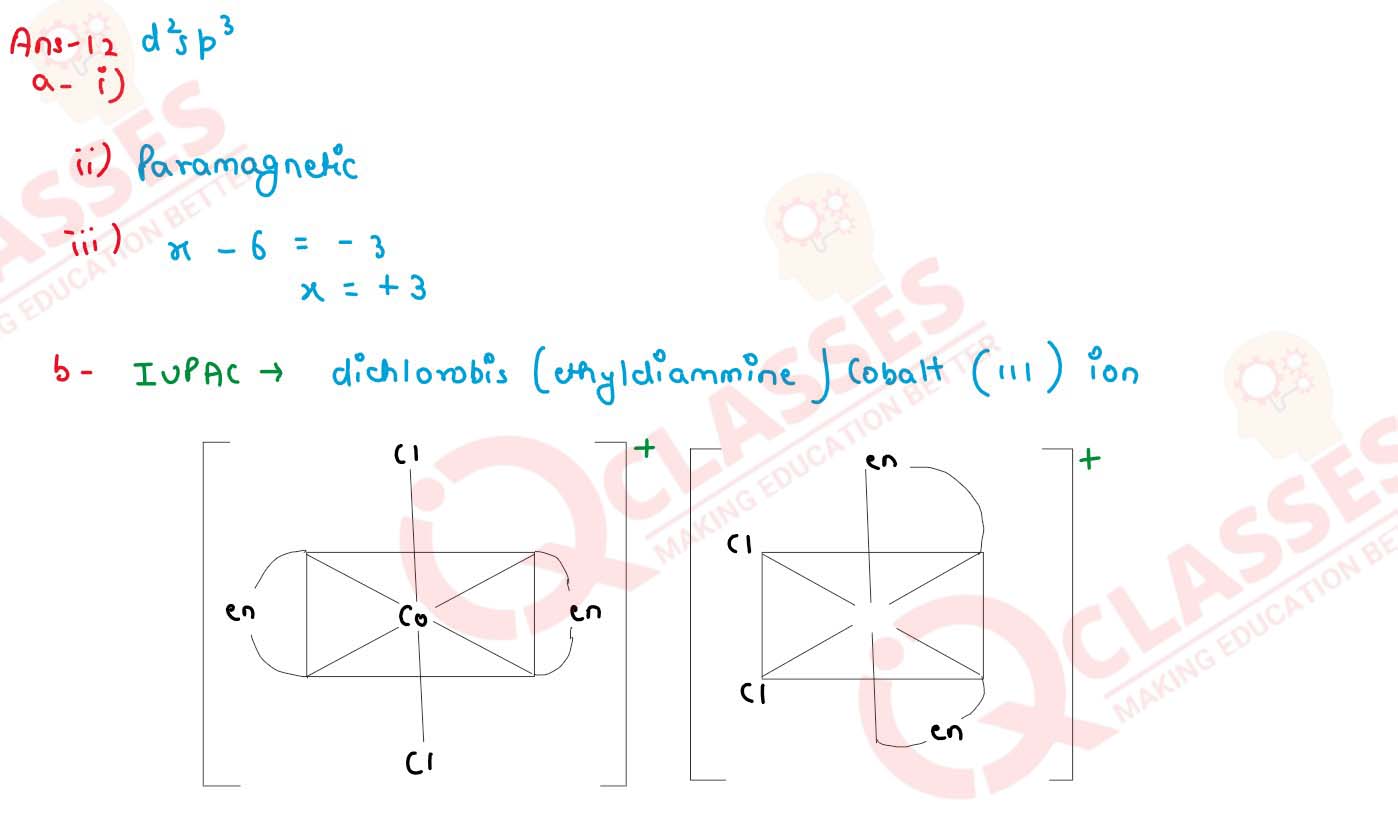
(i) the type of hybridisation.
(ii) the magnetic behavoiur.
(iii) the oxidation number of the central metal atom.
(b) Write the IUPAC name of [Co(en)2Cl2]+ ion and draw geometrical isomerism.
solutions

2017
Q4
(a) Write the formula of the following:
(i) potassium trioxalatoalluminate (III)
(ii) hexaaquairon (II) sulphate
(b) Name the type of isomerism shown by the following pairs of compounds :
(i) [Cu(NH3)4][PtCl4] and [Pt(NH3)4][CuCl4]
(ii) [Co(Pn)2Cl2]+ and [Co(tn)2Cl2]+
(c) For the coordination complex iron [Co (NH3)6]3+
(i) give the IUPAC name of the complex ion
(ii) what is the oxidation number of cobalt in the complex ion?
solutions
(i) potassium trioxalatoalluminate (III)
(ii) hexaaquairon (II) sulphate
(b) Name the type of isomerism shown by the following pairs of compounds :
(i) [Cu(NH3)4][PtCl4] and [Pt(NH3)4][CuCl4]
(ii) [Co(Pn)2Cl2]+ and [Co(tn)2Cl2]+
(c) For the coordination complex iron [Co (NH3)6]3+
(i) give the IUPAC name of the complex ion
(ii) what is the oxidation number of cobalt in the complex ion?
solutions

2016
Q5
(a) Write the upsc name of the following:
(i) [CO(NH3)4SO4]NO3
(ii) K[Pt(NH3)Cl3]
(b) What type of isomerism is exhibited by the following pairs of compound
(i)[PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2
(ii) [Cr(SCN)(H2O)5]2+ and [Cr(NCS)(H2O)5]2+
(c) How does K2[Pt Cl4] get ionised when dissolved in water? Will it form precipitate with AgNO3 solution is added to it? Give a reason for your answer
solutions
(i) [CO(NH3)4SO4]NO3
(ii) K[Pt(NH3)Cl3]
(b) What type of isomerism is exhibited by the following pairs of compound
(i)[PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2
(ii) [Cr(SCN)(H2O)5]2+ and [Cr(NCS)(H2O)5]2+
(c) How does K2[Pt Cl4] get ionised when dissolved in water? Will it form precipitate with AgNO3 solution is added to it? Give a reason for your answer
solutions



Add a comment