Class 12 Chemistry ISC D&F Block Board Questions
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter D&F Block. These important notes,board questions and predicted questions are based on ISC board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
2020
Q1
Explain the following:
(i) Transition metals and their compounds generally exhibit a paramagnetic behavior,
(ii) There is an increase in density of elements from titanium (Z=22) to copper (Z= 29) in the 3d series of transition elements.
(iii) K2Cr2O7 acts as a powerful oxidising agent in acidic medium.
solutions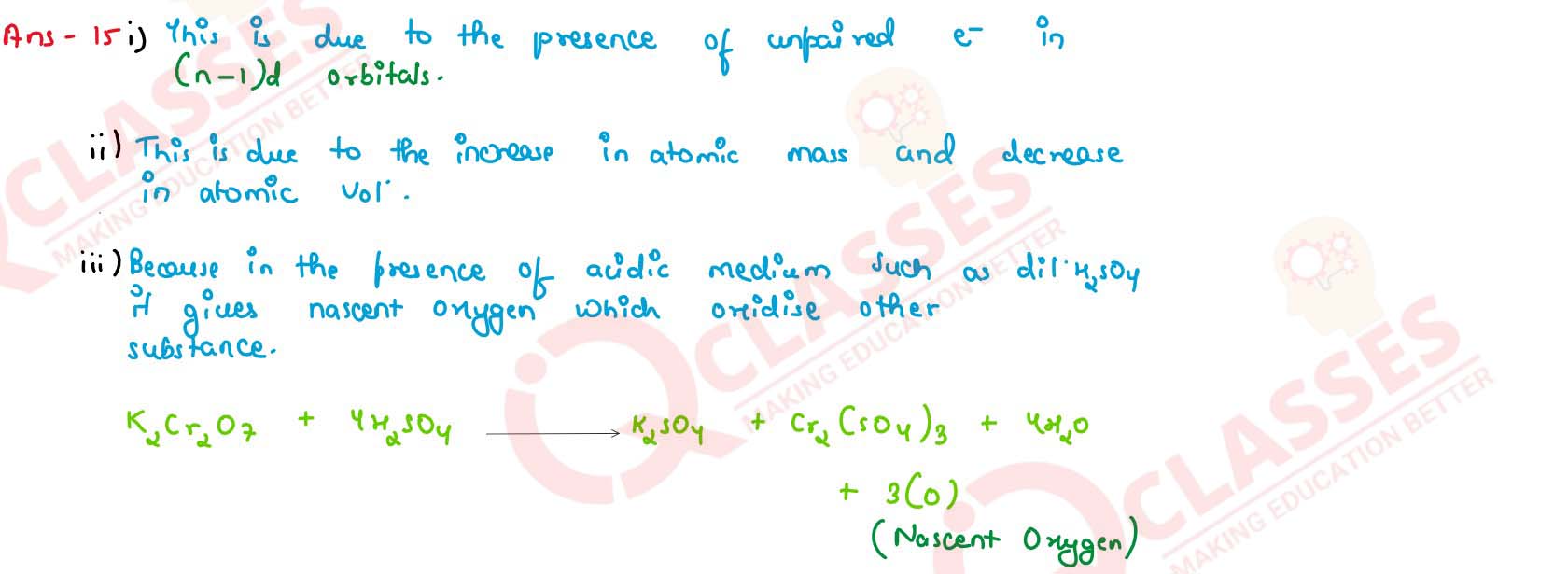
(i) Transition metals and their compounds generally exhibit a paramagnetic behavior,
(ii) There is an increase in density of elements from titanium (Z=22) to copper (Z= 29) in the 3d series of transition elements.
(iii) K2Cr2O7 acts as a powerful oxidising agent in acidic medium.
solutions

2019
Q2
Answer the following questions:
(i) (1) Which trivalent iron has maximum size in the lanthanoide series i.e. lanthanum ion (La3+) to lutetium ion (Lu3+) ? (Atomic number of lanthanum =57 and Lutetium= 71)
(2) Explain why Cu2+ is paramagnetic but Cu- is diamagnetic (atomic number of copper = 29)
solutions
(i) (1) Which trivalent iron has maximum size in the lanthanoide series i.e. lanthanum ion (La3+) to lutetium ion (Lu3+) ? (Atomic number of lanthanum =57 and Lutetium= 71)
(2) Explain why Cu2+ is paramagnetic but Cu- is diamagnetic (atomic number of copper = 29)
solutions

Q3
(a) Explain why :
(i) transition elements form alloys
(ii) Zn2+ Salts are white whereas Cu2+ salts are coloured
(iii) Transition metals and their compounds act as catalyst
solutions
(i) transition elements form alloys
(ii) Zn2+ Salts are white whereas Cu2+ salts are coloured
(iii) Transition metals and their compounds act as catalyst
solutions

OR
Q4
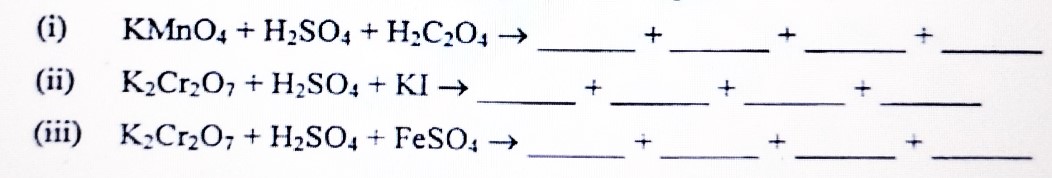
(b) Complete and balance the following chemical equations:
solutions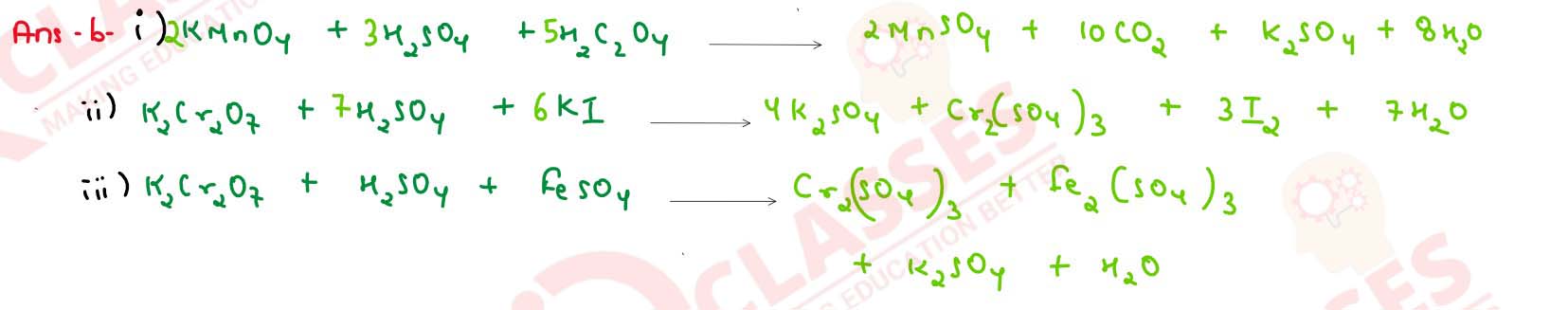

solutions

2018
Q5
Answer the following question:
(1) why does the density of transition element increase from Titanium to Copper? (Atomic number Ti= 22, Cu = 29)
(2) Why is zinc not regarded as a transition element? (At. no. of Zn = 30)
solutions
(1) why does the density of transition element increase from Titanium to Copper? (Atomic number Ti= 22, Cu = 29)
(2) Why is zinc not regarded as a transition element? (At. no. of Zn = 30)
solutions

Q6
(a) Explain why :
(i) Mn2+ is more stable than Fe2+ towards oxidation to +3 state.(Atomic no. Mn=25, Fe=26)
(ii) transition element usually form coloured ions
(iii) Zr and Hf exhibit similar properties. (At. no. of Zr=40, Hf=72)
solutions
(i) Mn2+ is more stable than Fe2+ towards oxidation to +3 state.(Atomic no. Mn=25, Fe=26)
(ii) transition element usually form coloured ions
(iii) Zr and Hf exhibit similar properties. (At. no. of Zr=40, Hf=72)
solutions

OR
Q7
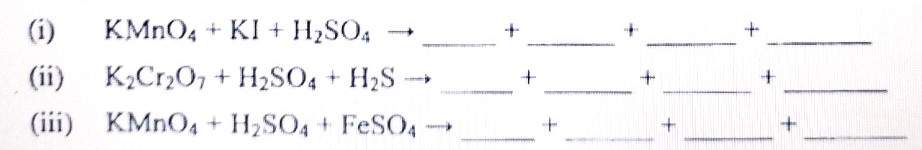
Complete and balance the following chemical equations :
solutions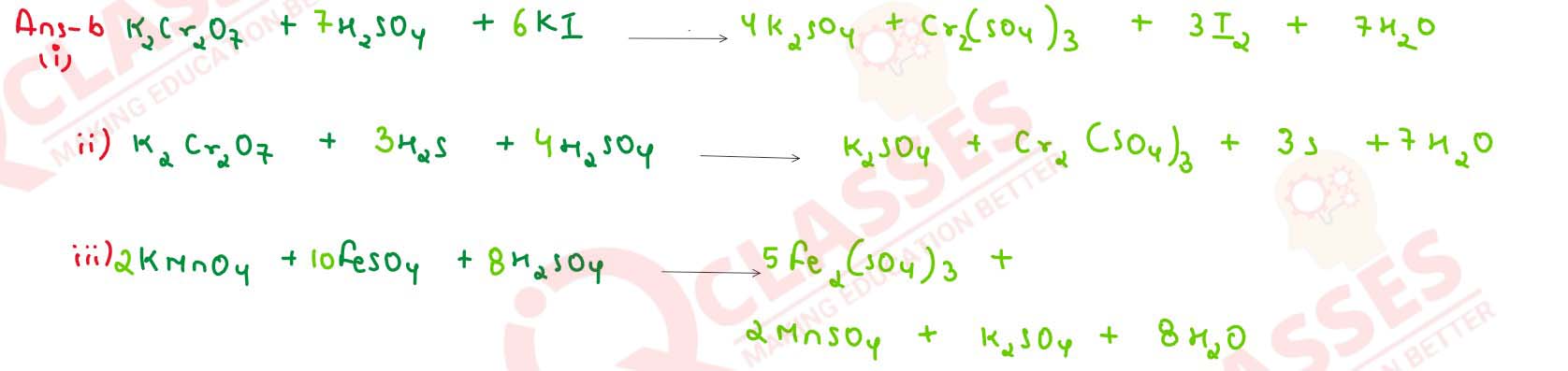

solutions

2017
Q8
Give balanced equation for the following reactions :
(i) potassium permanganate is heated with concentrated hydrochloric acid
(ii) lead sulphide is heated with hydrogen peroxide
(iii) ozone is treated with potassium iodide solution.
solutions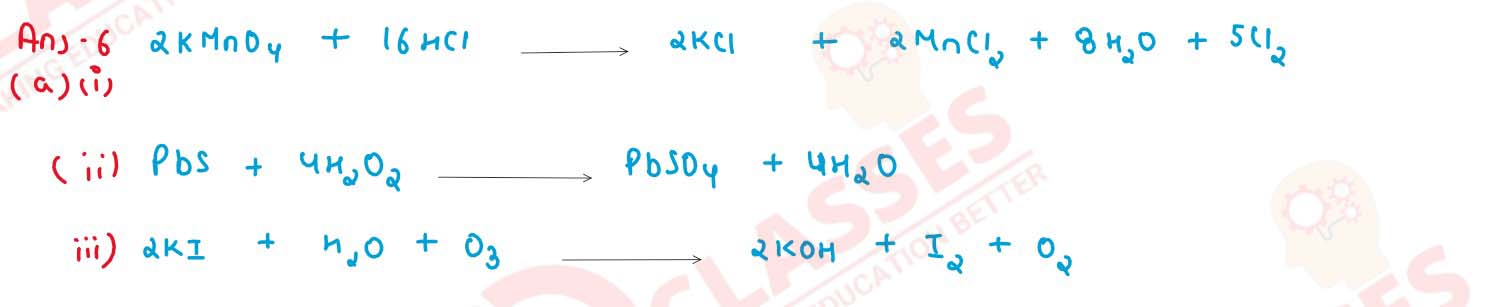
(i) potassium permanganate is heated with concentrated hydrochloric acid
(ii) lead sulphide is heated with hydrogen peroxide
(iii) ozone is treated with potassium iodide solution.
solutions

2016
Q9
(a) Give balanced equation for the following reaction:
potassium dichromate is treated with acidified ferrous sulphate solution
(b) How will you obtain pure potassium permanganate (KMnO4) crystals from its ore, pyrolusite ? Give the steps involved and the reactions
solutions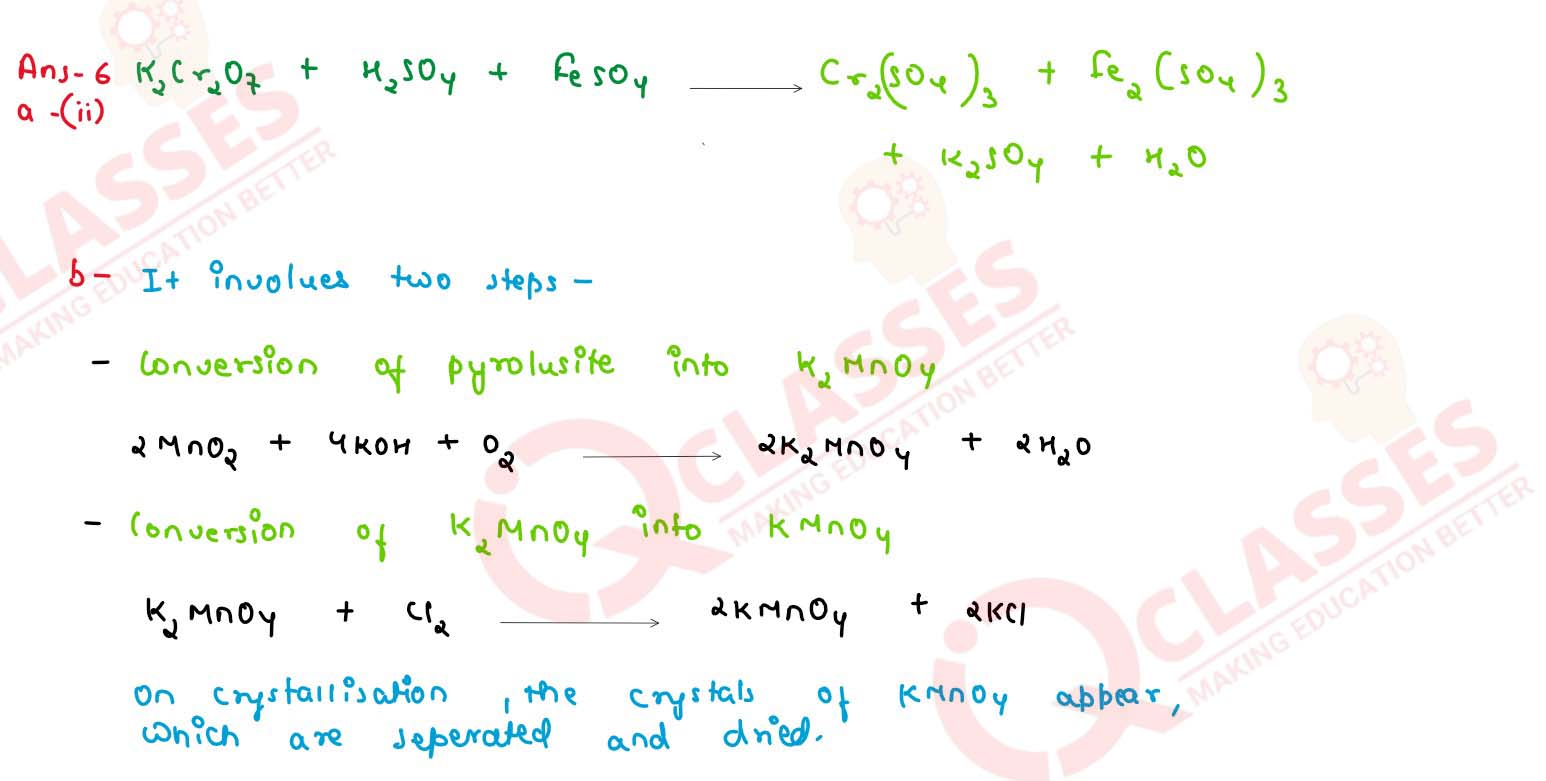
potassium dichromate is treated with acidified ferrous sulphate solution
(b) How will you obtain pure potassium permanganate (KMnO4) crystals from its ore, pyrolusite ? Give the steps involved and the reactions
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment