Class 12 Chemistry ISC Electrochemistry Mostlikely QuestionBank
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter Electrochemistry. These important notes,board questions and predicted questions are based on ISC board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
Q1
Define fuel cell and write its two advantages
solutions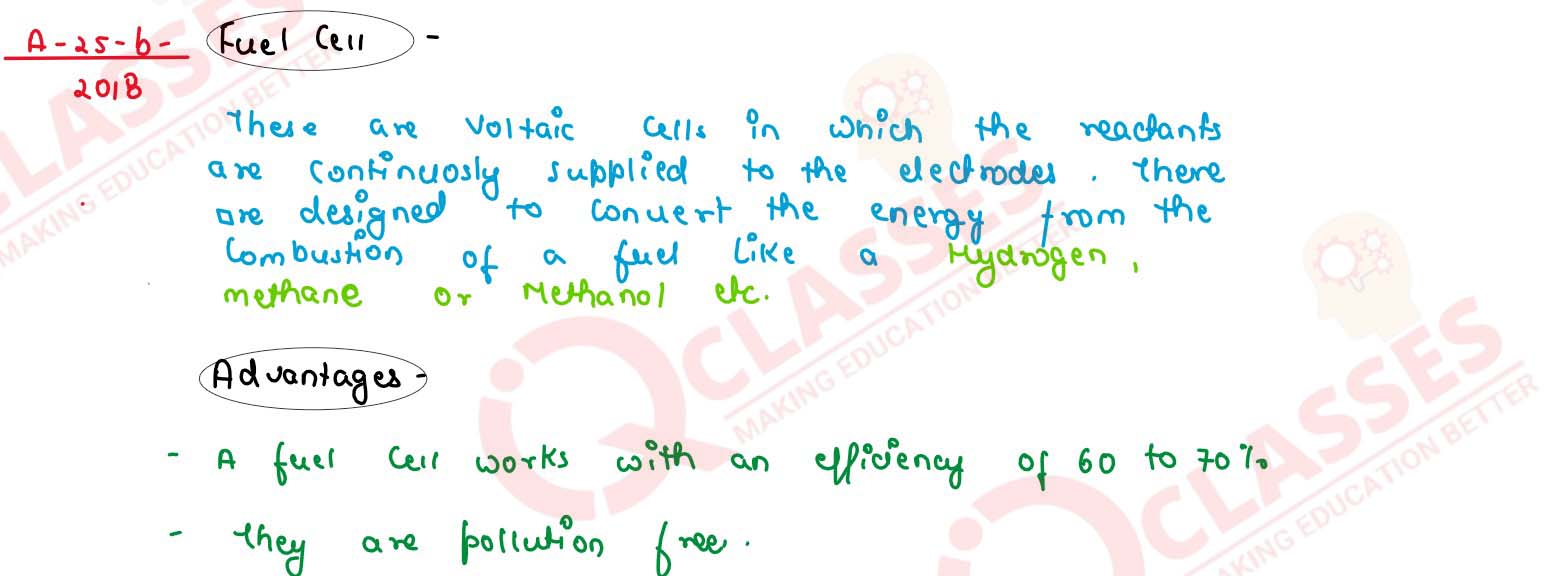
solutions

Q2
Define the following terms:
(a) Specific conductance
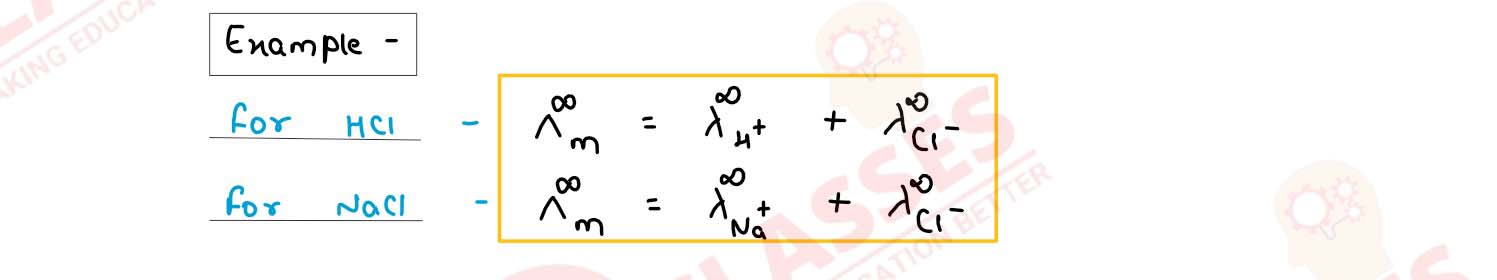
(b) Kohlrausch's law
solutions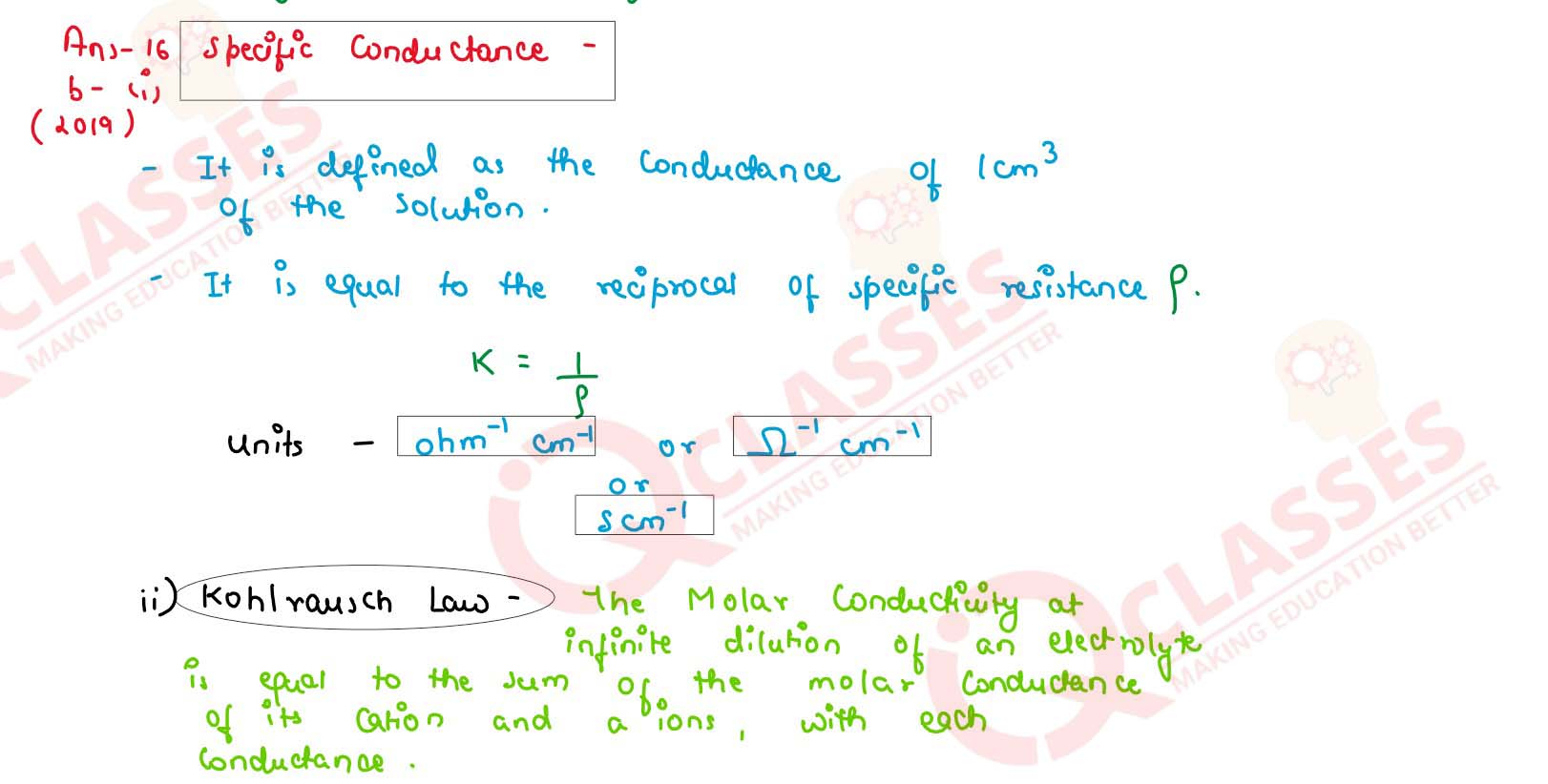
(a) Specific conductance
(b) Kohlrausch's law
solutions


Q3
The resistance of conductivity cell containing 0.01 M KCl solution at 298 K is 1500 Ω. What is
the cell constant if the conductivity of 0.001 M KCl solution add 298 K is 0.146 x 10-3 S
cm-1 ?
solutions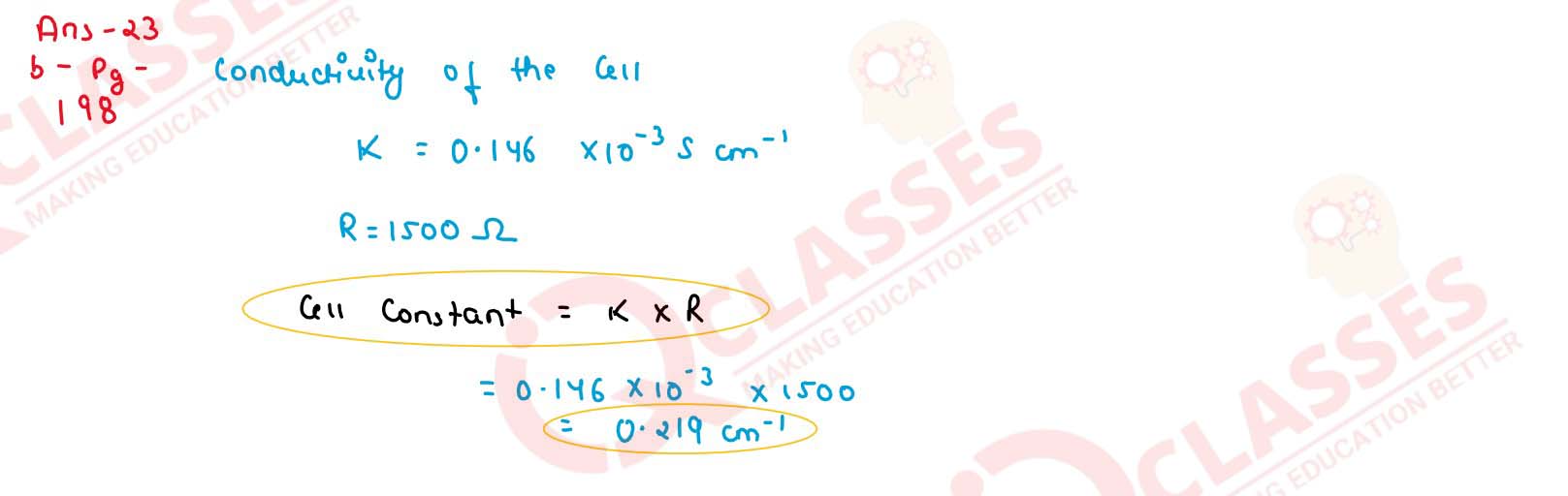
solutions
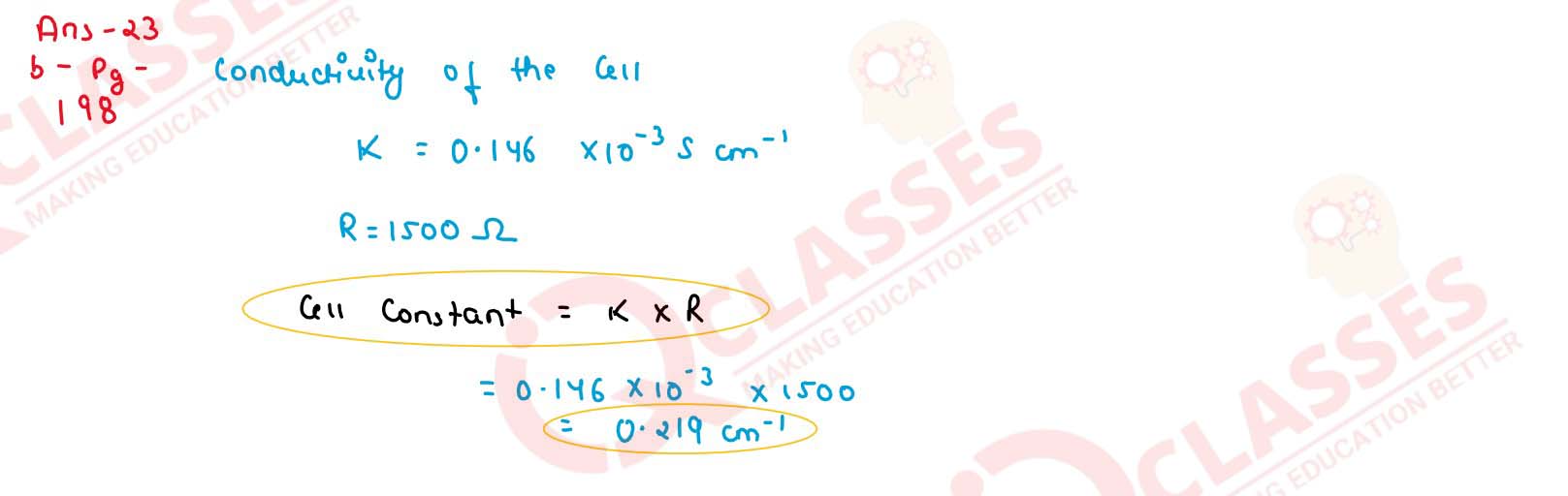
Q4
(a) The conductivity of 0.01 mol L-1 solution of CH2COOH is 3.905 x 10
-5 S cm-1 . Calculate its molar conductivity and degree of dissociation
(λ)
Given λo(H+) = 349.6 S cm2 mol-1 and λo(CH3COOH-) = 40.9 S cm2 mol-1
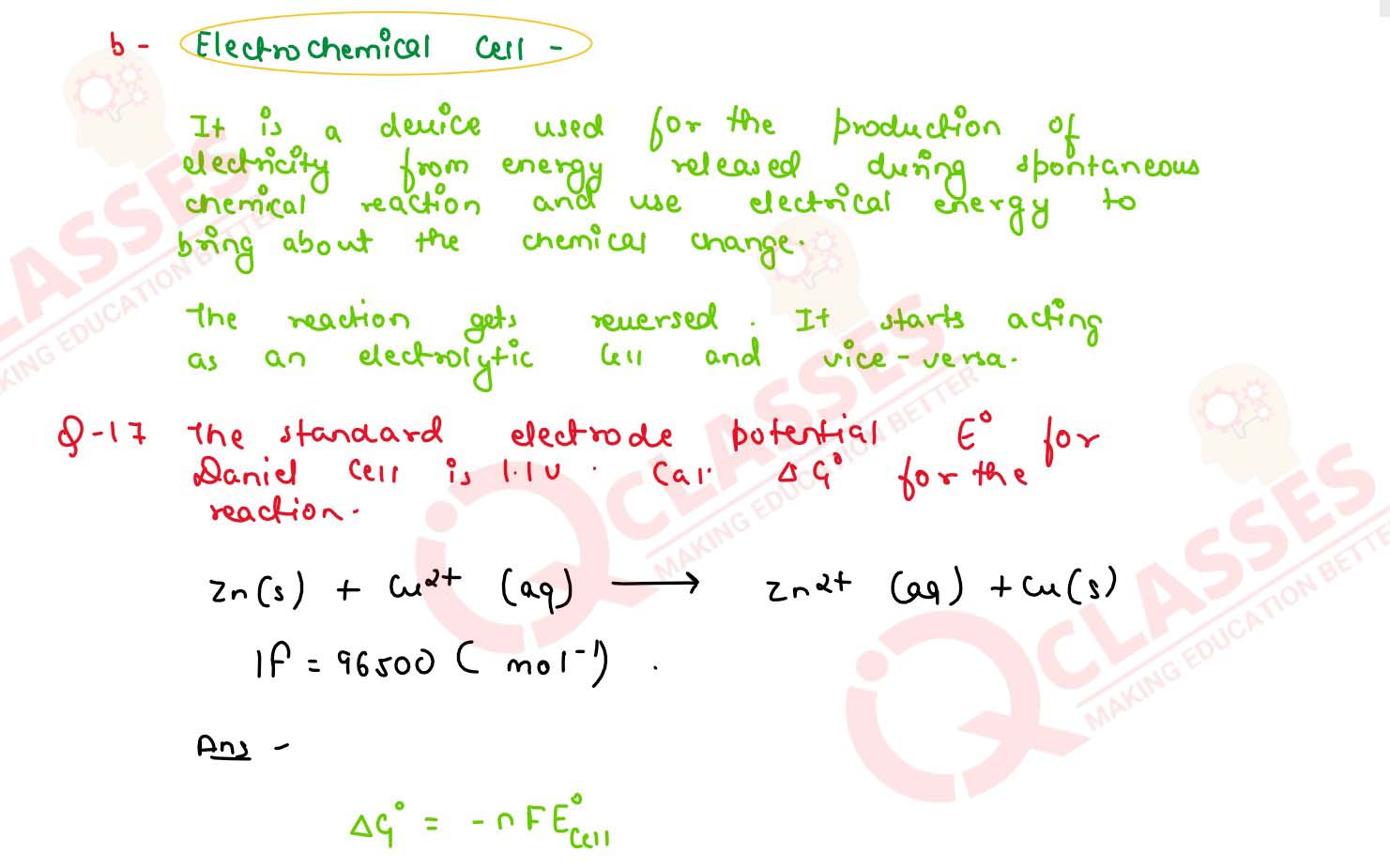
(b) Define electrochemical cell. What happens if external potential applied becomes greater than Eocell of electrochemical cell ?
solutions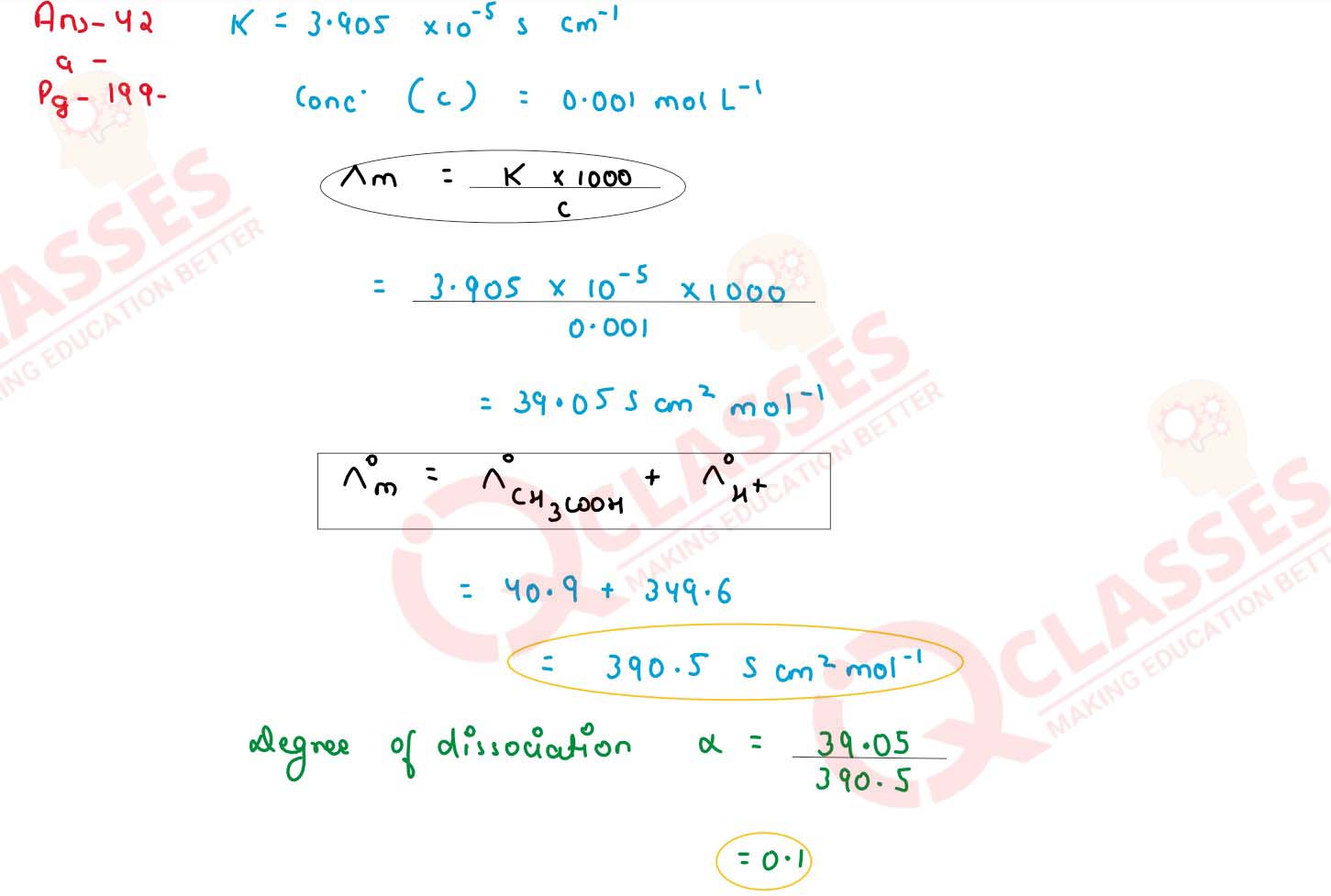

Given λo(H+) = 349.6 S cm2 mol-1 and λo(CH3COOH-) = 40.9 S cm2 mol-1
(b) Define electrochemical cell. What happens if external potential applied becomes greater than Eocell of electrochemical cell ?
solutions




Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment