Class 12 Chemistry ISC Solutions Mostlikely QuestionBank
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter solutions. These important notes,board questions and predicted questions are based on ISC board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
Q1
Define the following terms:
(i) Azeotrope
(ii) osmotic pressure
(iii) colligative properties
solutions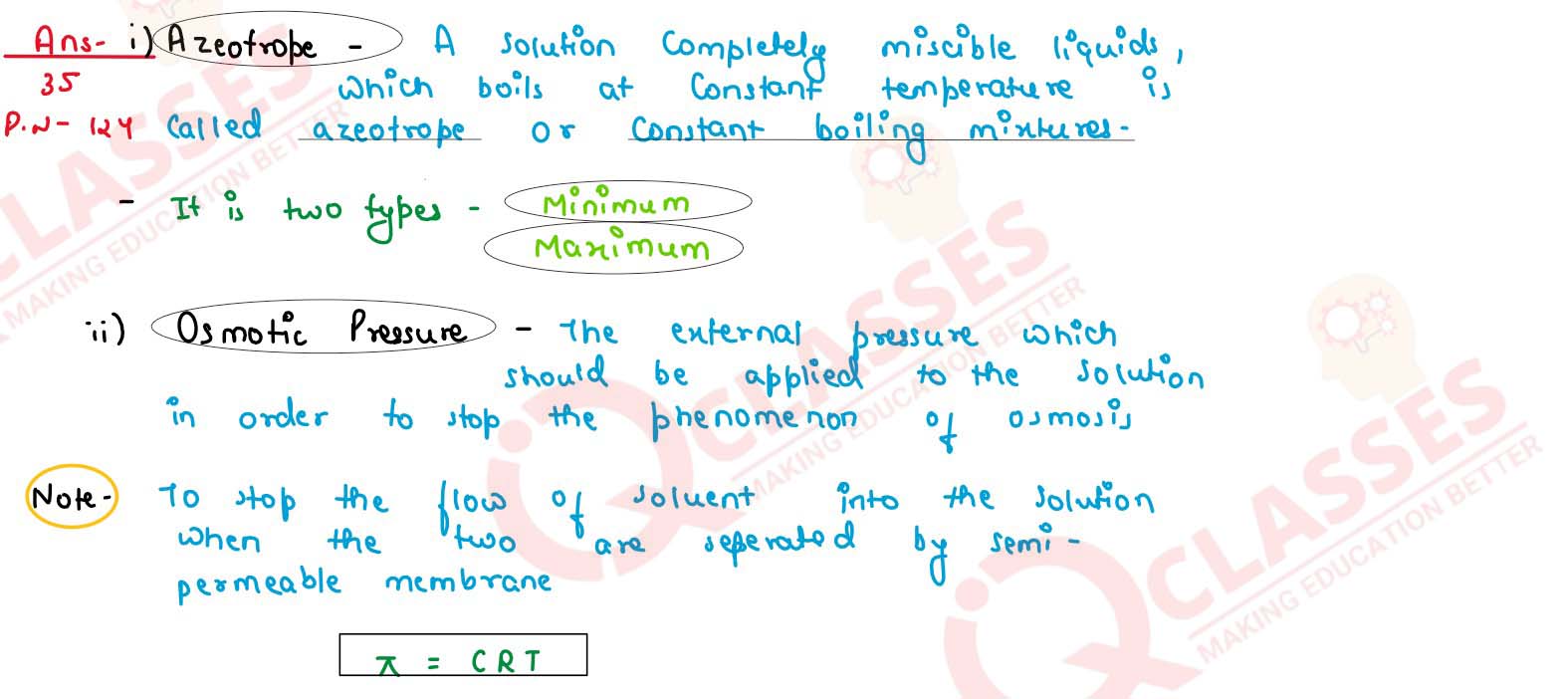

solutions


Q2
3.9 g benzoic acid dissolve in 49 g benzene shows or depression in freezing point of 1.62 K.
Calculate the van't Hoff factor and predict the nature of the solute (associated or dissociated)
(Given : Molar mass of benzoic acid = 122 g mol-1, Kf for benzene = 4.9 K kg mol-1)
solutions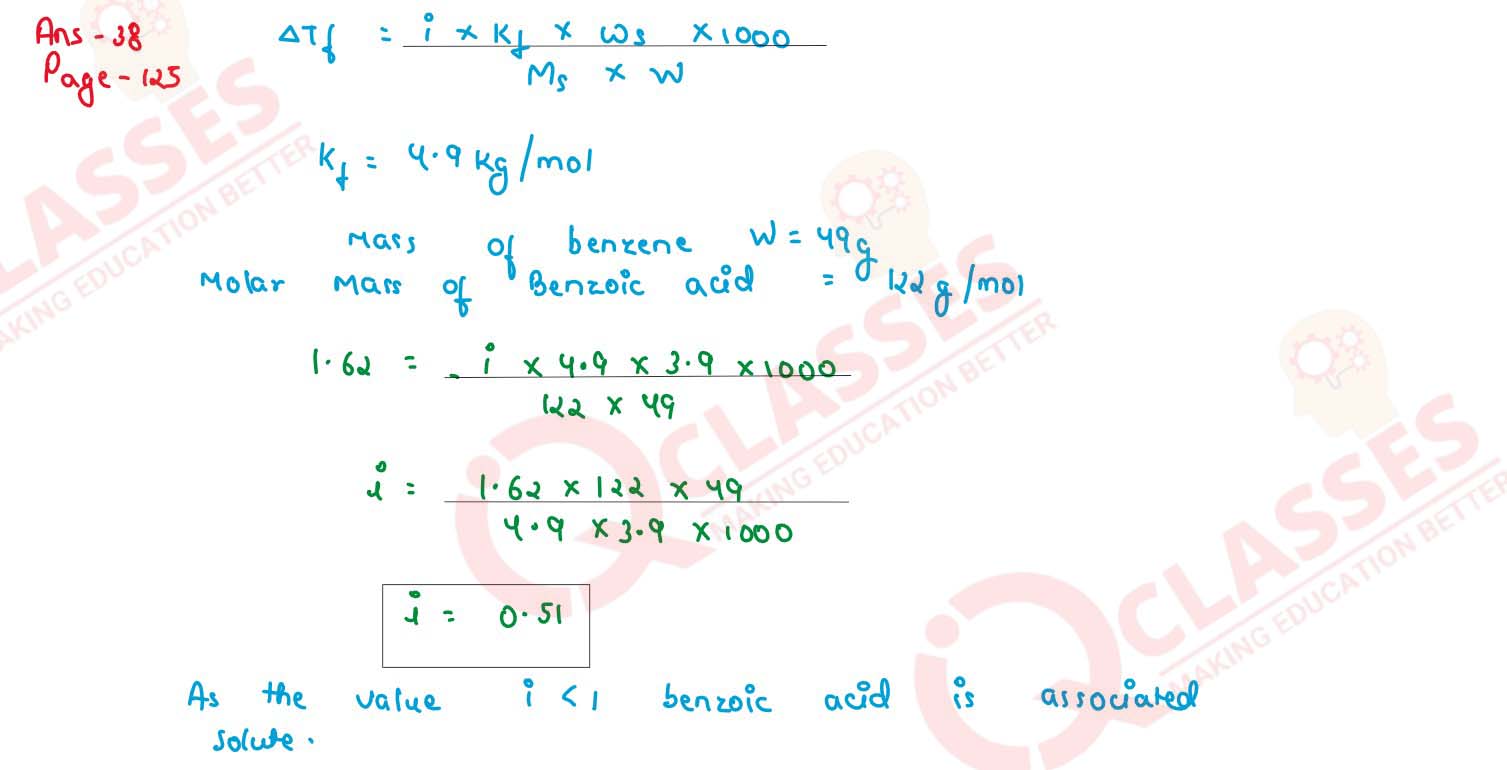
(Given : Molar mass of benzoic acid = 122 g mol-1, Kf for benzene = 4.9 K kg mol-1)
solutions

Q3
A solution is prepared by dissolving 10 g of non volatile solute in 200 g of water. It has a vapour
pressure of 31.84 mm Hg at 308 K. Calculate the molar mass of the solute.(vapour pressure of pure
water at 300 K is 32 mm Hg)
solutions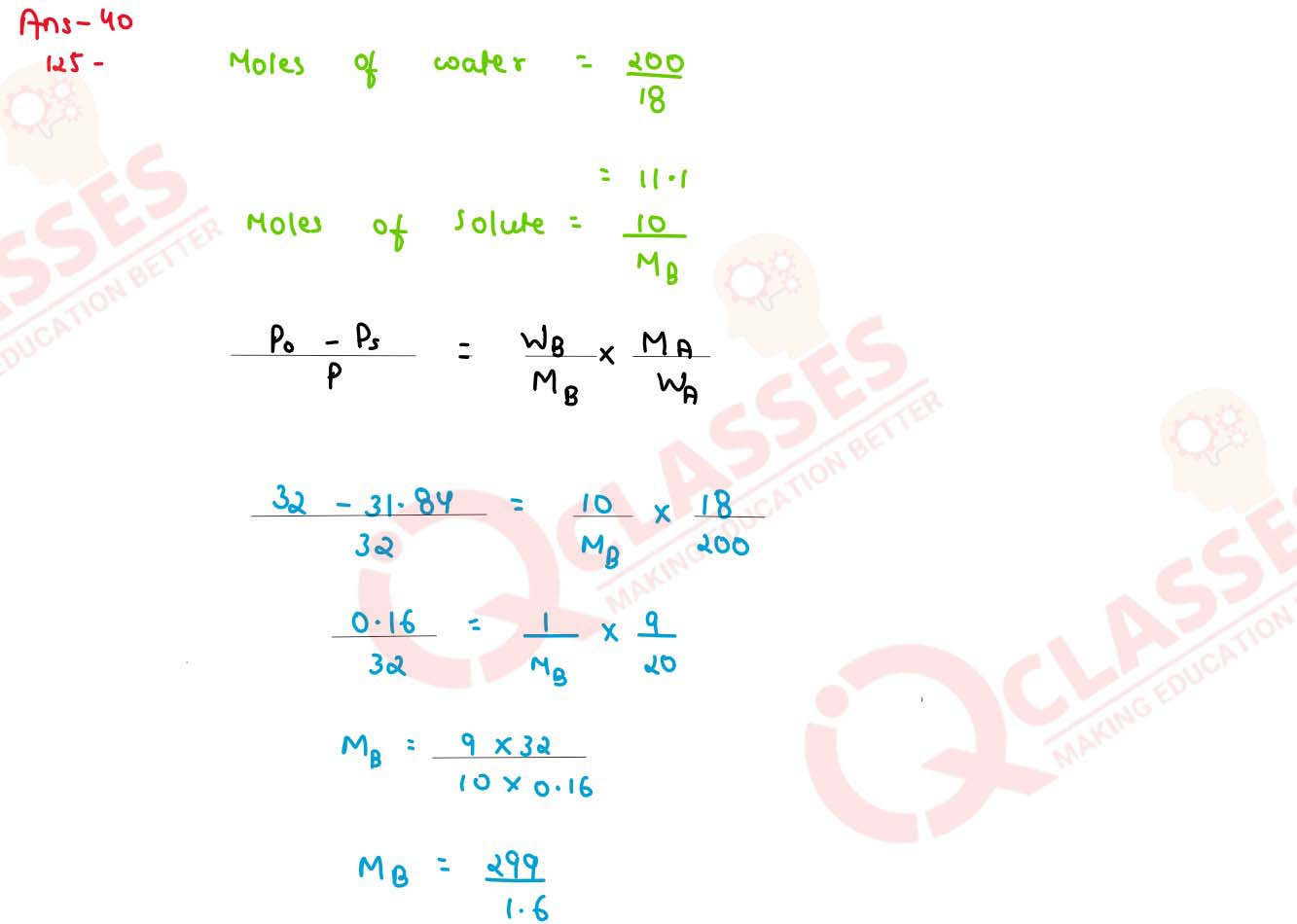

solutions


Q4
Calculate the boiling point of solution when 4 g of MgSO4 (M = 120 g mol-1)
was dissolved in 100 g of water assuming MgSO4 undergoes complete ionization.
(Kb for water = 0.52 kg mol-1 )
solutions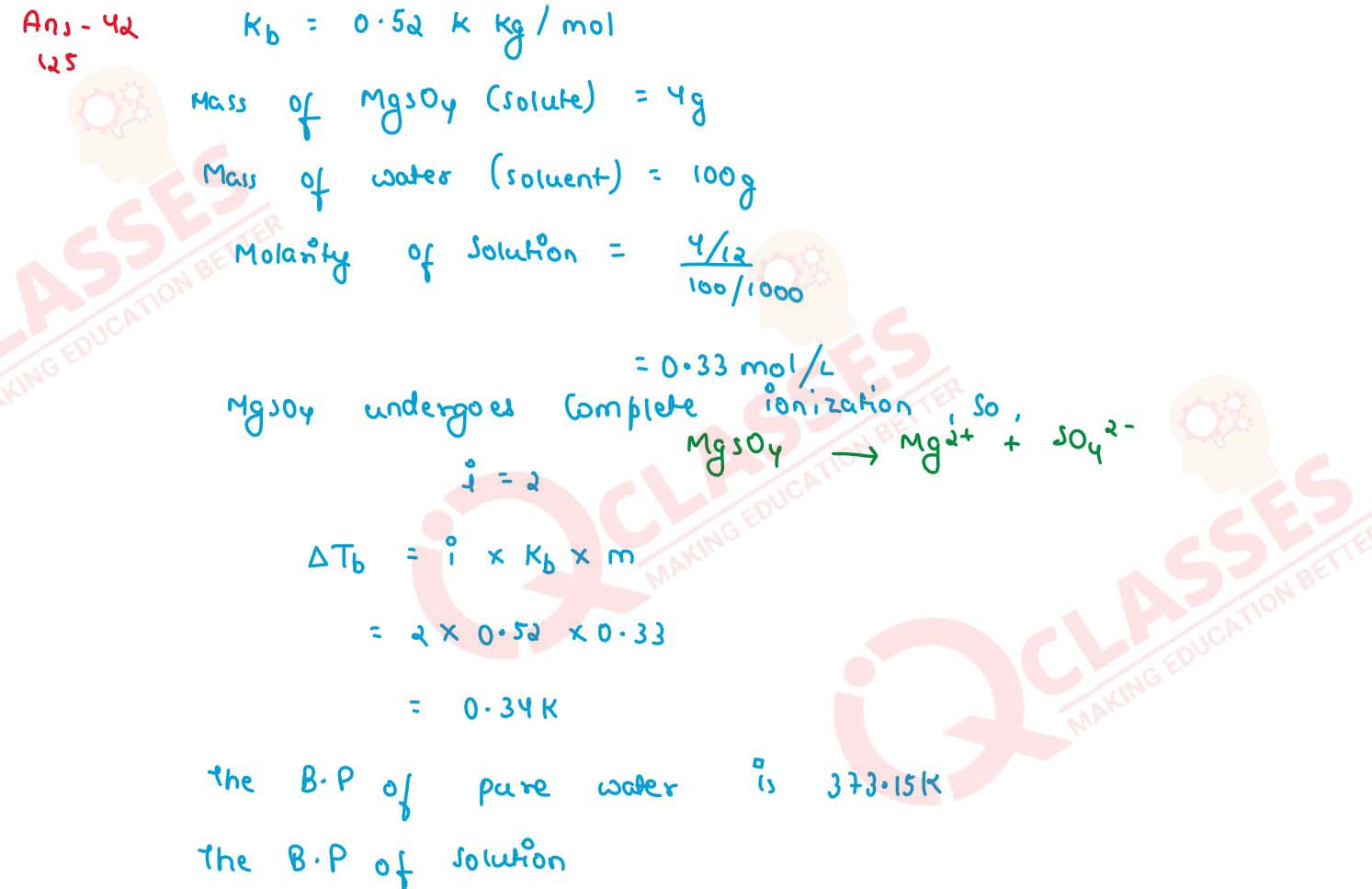
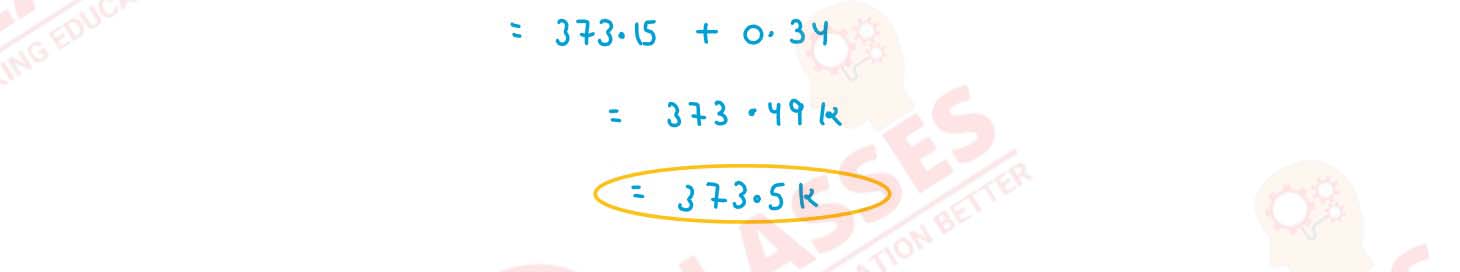
(Kb for water = 0.52 kg mol-1 )
solutions

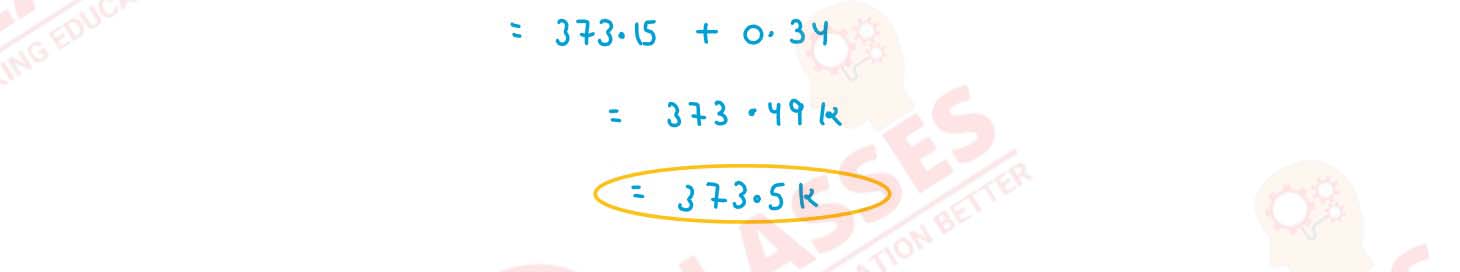


Add a comment