Class 10 ICSE Physics Radioactivity Boards Questions
Here we provide Class 10 physics important notes,board questions and predicted questions with Answers for chapter Radioactivity. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 physics syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
2020
Q1
(i) Differentiate between nuclear fusion and nuclear fission
(ii) State one safety precaution in the disposal of nuclear waste
solutions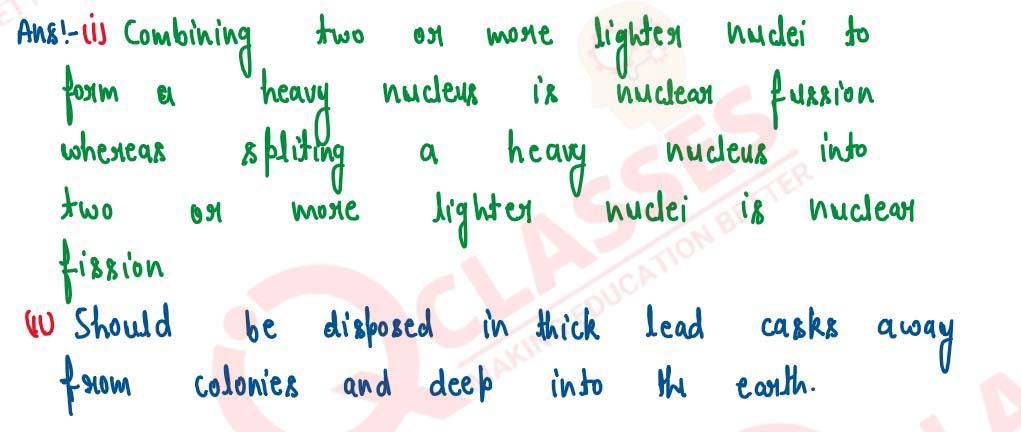
(ii) State one safety precaution in the disposal of nuclear waste
solutions

Q2
An atomic nucleus A is composed of 84 protons and 128 neutrons. The nucleus A emits an alpha panicle
and is transformed into a nucleus B.
(i) What is the composition of B?
(ii) The nucleus B emits a beta panicle and is transformed into a nucleus C. What is the composition of C?
(iii) What is mass number of the nucleus A?
(iv) Does the composition of C change if it emits gamma radiations?
solutions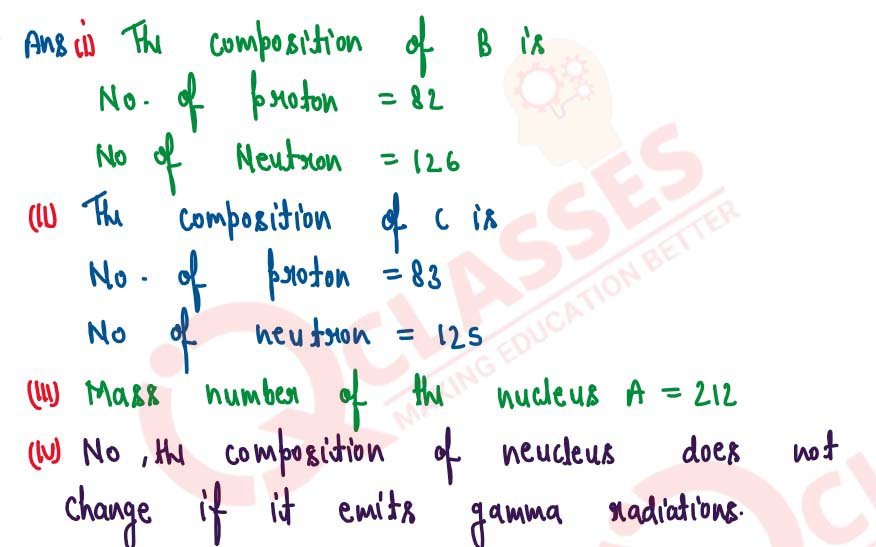
(i) What is the composition of B?
(ii) The nucleus B emits a beta panicle and is transformed into a nucleus C. What is the composition of C?
(iii) What is mass number of the nucleus A?
(iv) Does the composition of C change if it emits gamma radiations?
solutions

2019
Q3
Is it possible for a hydrogen (11H) nucleus to emit an alpha particle? Give a
reason for your answer.
solutions
solutions

Q4
(i) Define nuclear fission.
(ii) Rewrite and complete the following nuclear reaction by lilting in the atomic number of Ba and mass number of Kr
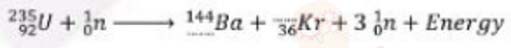
solutions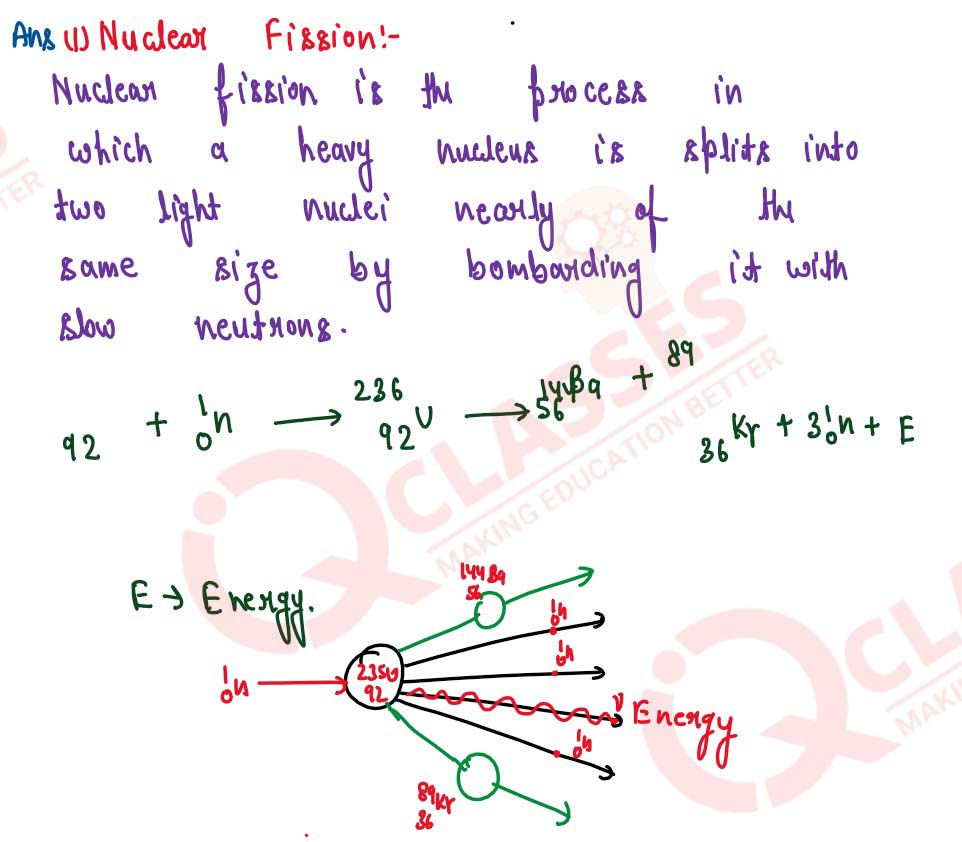
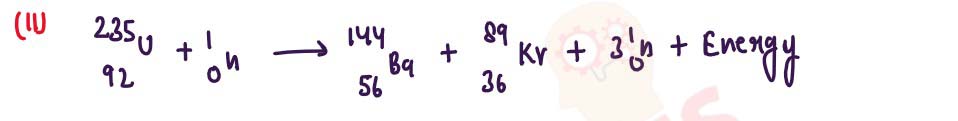
(ii) Rewrite and complete the following nuclear reaction by lilting in the atomic number of Ba and mass number of Kr
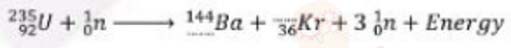
solutions

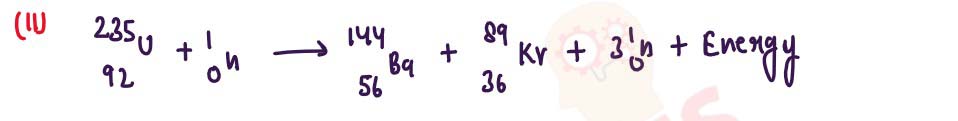
2018
Q5
(i) What do you understand by the term nuclear fusion?
(ii) Nuclear power plants use nuclear fission reaction to produce electricity. What is the advantage of producing electricity by fusion reaction?
solutions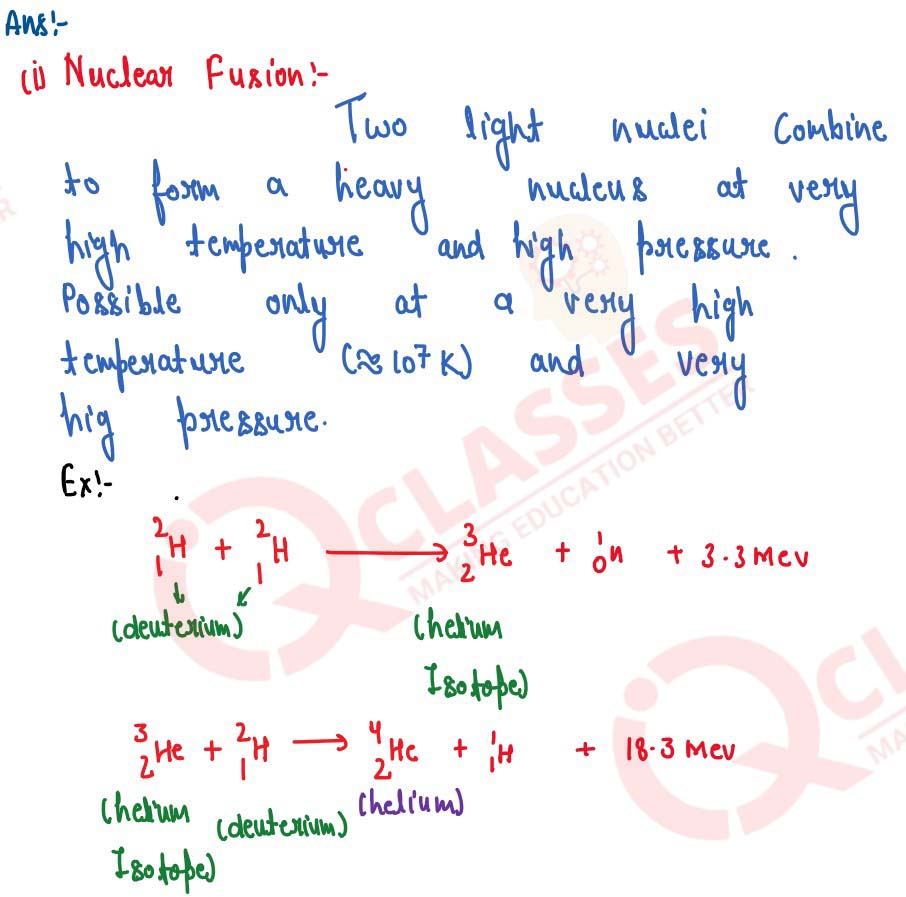

(ii) Nuclear power plants use nuclear fission reaction to produce electricity. What is the advantage of producing electricity by fusion reaction?
solutions


Q6
The ore of Uranium found in nature contains 92U238 and
92U235. Although both the isotopes are fissionable, it is found out
experimentally that one of the two isotopes is more easily fissionable.
(i) Name the isotope of Uranium which is easily fissionable.
(ii) Give a reason for your answer.
(iii) Write a nuclear reaction when Uranium 238 emits an alpha particle to form a Thorium (Th) nucleus.
solutions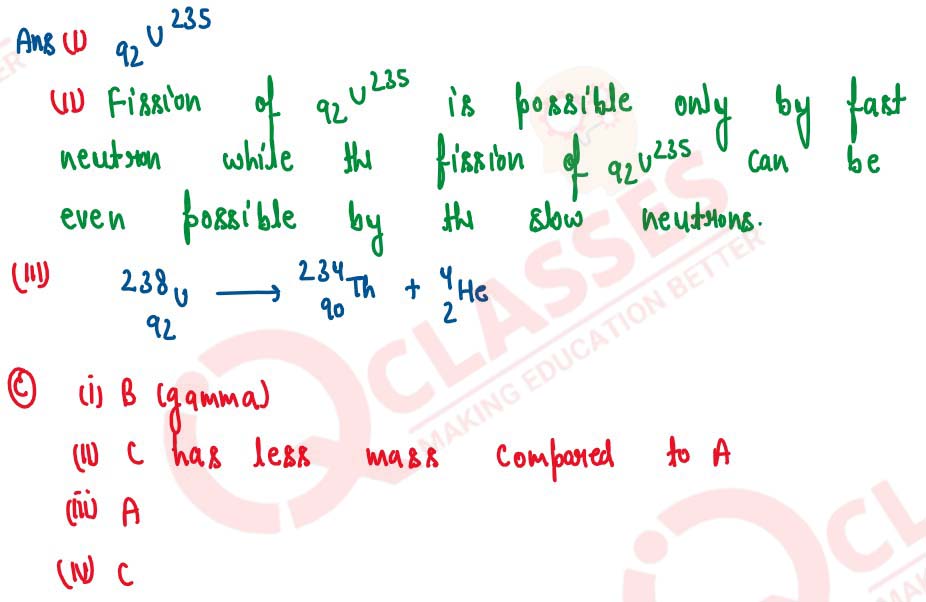
(i) Name the isotope of Uranium which is easily fissionable.
(ii) Give a reason for your answer.
(iii) Write a nuclear reaction when Uranium 238 emits an alpha particle to form a Thorium (Th) nucleus.
solutions

2017
Q7
State two factors upon which the rate of emission of thermions depends.
solutions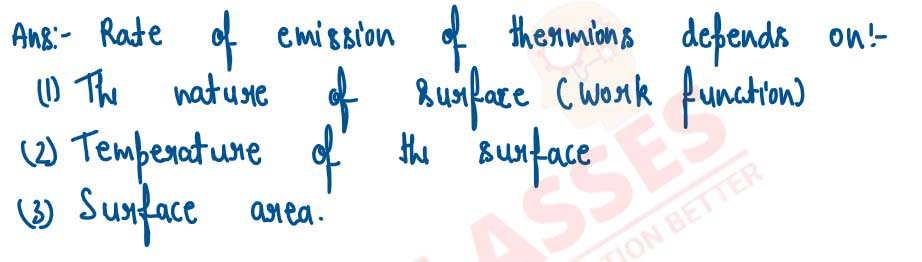
solutions

Q8
When does the nucleus of an atom tend to be radioactive ?
solutions
solutions

Q9
State 2 characteristics of the good thermion emitter
solutions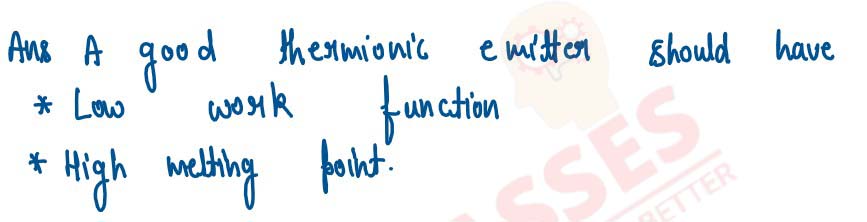
solutions

Q10
(i) Name the radiations which arc absorbed by greenhouse gases in the earth's atmosphere.
(ii) A radiation X is focused by a particular device on the bulb of a thermometer and mercury in the thermometer shows a rapid increase. Name the radiation X.
(iii) Name two factors on which the heat energy liberated by a body depends.
solutions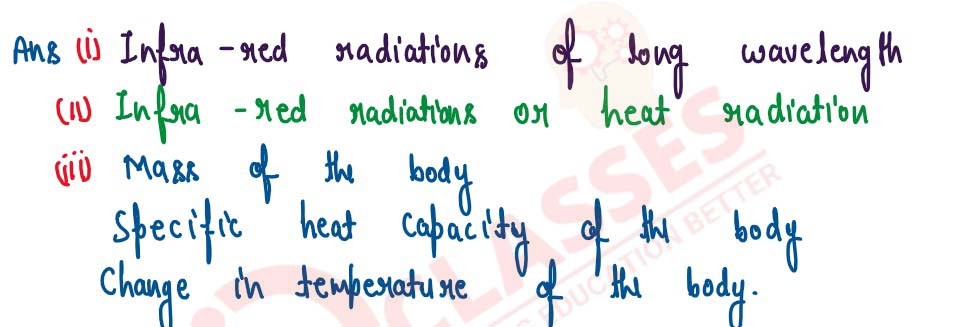
(ii) A radiation X is focused by a particular device on the bulb of a thermometer and mercury in the thermometer shows a rapid increase. Name the radiation X.
(iii) Name two factors on which the heat energy liberated by a body depends.
solutions

Q11
(i) What are free electrons?
(ii) Why do they not leave the metal surface on their own?
(iii) How can they be made to leave the metal surface? (State any two ways)
solutions
(ii) Why do they not leave the metal surface on their own?
(iii) How can they be made to leave the metal surface? (State any two ways)
solutions

2016
Q12
State the characteristics required of the good thermion emitter
solutions
solutions

Q13
Arrange α, β and γ rays in ascending order with respect to their
(i) Penetrating power.
(ii) Ionising power.
(iii) Biological effect.
solutions
(i) Penetrating power.
(ii) Ionising power.
(iii) Biological effect.
solutions

Q14
(i) In a cathode ray tube what is the function of anode?
(ii) State the energy conversion taking place in a cathode ray tube.
(iii) Write one use of cathode ray tube.
solutions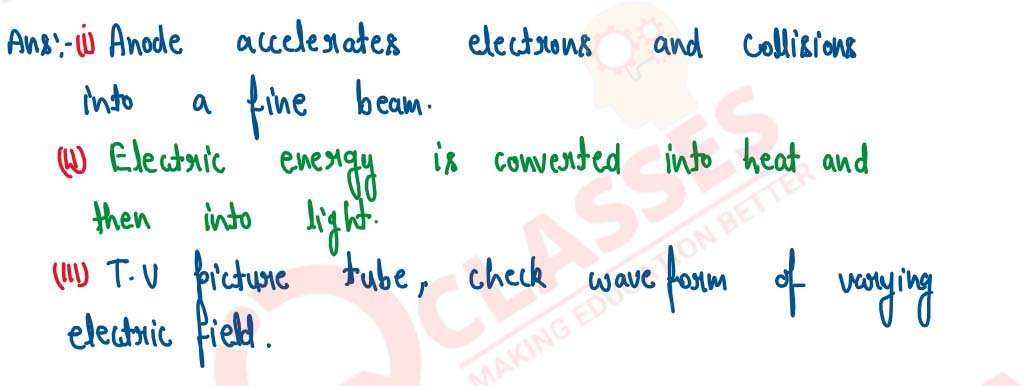
(ii) State the energy conversion taking place in a cathode ray tube.
(iii) Write one use of cathode ray tube.
solutions

Q15
(i) Represent the change in the nucleus of a radioactive element when a β particle is
emitted.
(ii) What is the name given to elements with same mass number and different atomic number?
(iii) Under which conditions does the nucleus of an atom tend to be radioactive?
solutions
(ii) What is the name given to elements with same mass number and different atomic number?
(iii) Under which conditions does the nucleus of an atom tend to be radioactive?
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment