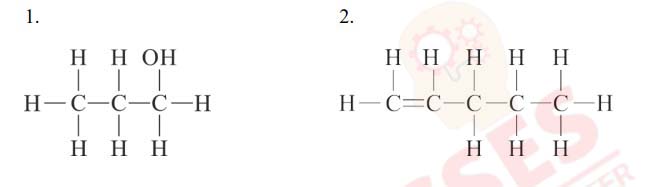Maximum Marks: 80
Time allowed: Two and half hours
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during first 15 minutes.
This time is to be spent in reading the question paper.
The time given at the head of this Paper is the time allowed for writing the answers.
Section-A
(Attempt all questions from this Section.)
Question 1
Choose one correct answer to the questions from the given options:
(i) An aqueous solution of copper sulphate turns colourless on electrolysis.
Which of the following could be the electrodes?
P. anode: copper; cathode: copper
Q. anode: platinum; cathode: copper
R. anode: copper; cathode: platinum
(a) only P
(b) only Q
(c) only R
(d) both Q and R
View
Solution
(ii) A compound P is heated in a test tube with sodium hydroxide solution. A red litmus
paper held at the mouth of the test tube turns blue.
Which of the following could compound P be?
(a) zinc sulphate
(b) copper sulphate
(c) ferrous sulphate
(d) ammonium sulphate
View
Solution
(iii) The atomic masses of sulphur (S), oxygen (O), and helium (He) are approximately
32, 16, and 4 respectively.
Which of the following statements regarding the number of atoms in 32 g of sulphur,
16 g of oxygen, and 4 g of helium is correct?
P. 16 g of oxygen contains four times the number of atoms as 4 g of helium.
Q. 16 g of oxygen contains half the number of atoms as 32 g of sulphur.
(a) only P
(b) only Q
(c) both P and Q
(d) neither P nor Q
View
Solution
(iv) Ammonia gas is passed through quicklime and then collected in a jar. Red and blue
litmus papers are placed in the jar. W, X, Y and Z are the four observations.
Which of the above observations correctly shows the reaction of the litmus papers
to ammonia?
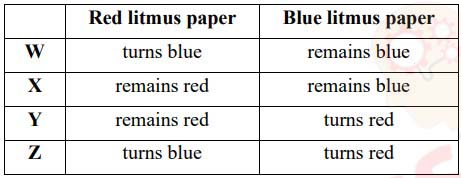
(a) W
(b) X
(c) Y
(d) Z
View
Solution
(v) Glucose reacts with concentrated sulphuric acid to give a very pure form of carbon
called sugar charcoal.
The reaction taking place is:
(a) oxidation
(b) combustion
(c) dehydration
(d) combination
View
Solution
(vi) In which of the following electrolytic cells [P, Q, R or S] will silver plating be done
on the spoon?
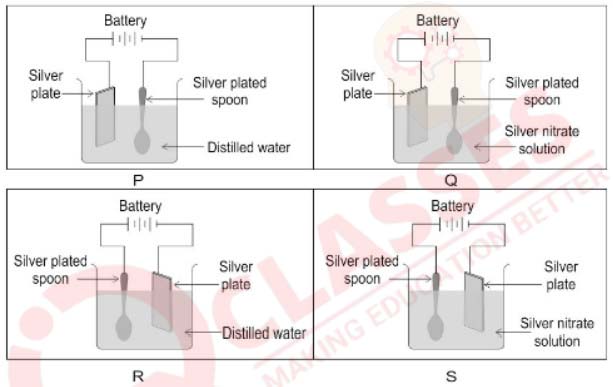
(a) P
(b) Q
(c) R
(d) S
View
Solution
(vii) The basicity of acetic acid is:
(a) 1
(b) 2
(c) 3
(d) 4
View
Solution
(viii) A → A+3; B → B-1
Number of electrons present in the outermost shell of atoms A and B respectively are:
(a) 5, 1
(b) 3, 1
(c) 3, 7
(d) 5, 7
View
Solution
(ix) A __________ solution is observed after placing Magnesium metal in a solution of
Copper sulphate for half an hour.
(a) Blue
(b) Colourless
(c) Reddish brown
(d) Dirty green
View
Solution
(x) An element with atomic no. __________ will form an acidic oxide.
(a) 3
(b) 17
(c) 11
(d) 13
View
Solution
(xi) Which of the following is NOT true with respect to nitric acid?
(a) It is a strong reducing agent
(b) It is a strong oxidizing agent
(c) It is unstable to heat
(d) It liberates sulphur dioxide gas when treated with potassium sulphite
View
Solution
(xii) __________ is the functional group in methanol.
(a) >C=O
(b) –OH
(c) –CHO
(d) –COOH
View
Solution
(xiii) The process of electrolysis is an example of:
(a) Oxidation reaction
(b) Reduction reaction
(c) Redox reaction
(d) Displacement reaction
View
Solution
(xiv) The catalyst used in Ostwald’s process is ___________.
(a) Finely divided iron
(b) Graphite
(c) Vanadium pentoxide
(d) Platinum
View
Solution
(xv) An element belongs to third period and sixteenth group. It will have __________
electrons in its valence shell.
(a) 2
(b) 5
(c) 6
(d) 3
View
Solution
Question 2
Section-B
(Attempt any four questions from this Section.)
Question 3
(i) Identify the reactant and write the balanced equation for the following:
Nitric acid reacts with compound Q to give a salt Ca(NO3)2, water and carbon
dioxide.
(ii) What property of Sulphuric acid is exhibited in each of the following cases:
(a) In the preparation of HCl gas when it reacts with Sodium chloride.
(b) When conc. Sulphuric acid reacts with Copper to produce Sulphur dioxide gas.
(iii) The electron affinity of an element X is greater than that of element Y.
(a) How is the oxidising power of X likely to compare with that of Y?
(b) How is the electronegativity of X likely to compare with that of Y?
(c) State whether X is likely to be placed to the left or to the right of Y in the
periodic table?
(iv) (a) State wether the following statements are TRUE or FALSE. Justify your
answer.
1. In an electrovalent compound, the cation attains the electronic
configuration of the noble gas that comes after it in the periodic table.
2. In the formation of a compound PQ2, atom P gives one electron to each
atom of Q. The compound PQ2 is a good conductor of electricity.
(b) Calculate the number of moles in 22 grams of carbon dioxide .
View
Solution
Section-B
(Attempt any four questions from this Section.)
Question 4
(i) The following questions relate to the extraction of Aluminium by electrolysis.
(a) Name the other aluminum containing compound added to alumina.
(b) Give a balanced equation for the reaction that takes place at the cathode.
(ii) A gas cylinder of capacity 40 dm3 is filled with gas X the mass of which is 20 g.
When the same cylinder is filled with hydrogen gas at the same temperature and
pressure the mass of hydrogen is 2 g. Find the relative molecular mass of the gas.
(iii) Give balanced equations for each of the following:
(a) Action of warm water on Aluminium nitride.
(b) Oxidation of carbon with conc. Nitric acid.
(c) Dehydration of ethanol by conc. Sulphuric acid at a temperature of 170o
C.
(iv) With respect to Haber’s process answer the following:
(a) Temperature of the reaction
(b) Catalyst used
(c) Balanced equation for the reaction occurring
View
Solution
Question 5
(i) (a) Ranjana wants to prove that ammonia is a reducing agent. To demonstrate this,
she passes ammonia gas over heated copper oxide. What will she observe?
(b) Write a balanced chemical equation for the above reaction.
(ii) Name the alloy which is made up of:
(a) Copper, Zinc and Tin
(b) Lead and Tin
(iii) Seema takes a blue crystalline salt P in a test tube. On heating it produces a white
anhydrous powder. P is dissolved in water. Zinc is added to one part of the solution
and to another part of the solution Barium chloride is added.
(a) Name the compound P.
(b) Mention one observation when zinc is added to the solution of P.
(c) State the colour of the precipitate formed when barium chloride is added to the
solution of P.
(iv) Give reasons:
(a) Ethene undergoes addition reaction.
(b) Hydrocarbons can be used as fuels.
(c) Hydrogen chloride gas cannot be collected over water.
View
Solution
Question 6
(i) Name the following:
(a) The ore of Zinc containing its sulphide .
(b) The most commonly used oxide ore of Aluminium.
(ii) State one observation in the following cases:
(a) Sodium chloride solution is added to a solution of lead nitrate.
(b) Barium chloride solution is added to a solution of Zinc sulphate.
(iii) Copper sulphate solution is electrolysed using copper electrodes.
(a) Which electrode [cathode or anode] is the oxidizing electrode? Why?
(b) Write the equation for the reaction occurring at the above electrode
(iv) X [2, 8, 7] and Y [2, 8, 2] are two elements. Using this information complete the
following:
(a) __________ is the metallic element.
(b) Metal atoms tend to have a maximum of __________ electrons in the
outermost shell.
(c) ___________ is the reducing agent
View
Solution
Question 7
(i) The empirical formula of an organic compound is C3H4N. Its molecular weight is 108.
Find the amount of carbon in one mole of the compound. Show all the steps involved.
(Atomic weights: C- 12; H- 1; N- 14)
(ii) (a) Mahesh prepared a basic solution X that has a pH 7.
How will the pH of the solution X change on addition of the following:
1. Hydrochloric acid
2. a solution of a base
(b) The atomic number of an element is 15. To which group will this element
belong to?
(iii) 8.2 grams of calcium nitrate is decomposed by heating according to the equation
2Ca(NO3)2 _____ → 2CaO + 4NO2 + O2
Calculate the following:
(a) Volume of nitrogen dioxide obtained at STP
(b) Mass of CaO formed
[Atomic weights: Ca –40 , N—14, O—16]
View
Solution
Question 8
(i) State giving reasons if:
(a) zinc and aluminium can be distinguished by heating the metal powder with
concentrated sodium hydroxide solution.
(b) calcium nitrate and lead nitrate can be distinguished by adding ammonium
hydroxide solution to the salt solution.
(ii) Draw the electron dot diagram of Hydronium ion.
(iii) Give balanced equations for the following:
(a) Laboratory preparation of ethyne from calcium carbide.
(b) Conversion of acetic acid to ethyl acetate.
(c) Laboratory preparation of nitric acid.
(iv) Identify the following substances:
(a) An alkaline gas which produces dense white fumes when reacted with HCl
gas.
(b) The anion present in the salt, which produces a gas with the smell of rotten
eggs when reacted with dil. HCl.
(c) The particles present in strong electrolytes.
View
Solution