Class 10 Chemistry ICSE Mole Mostlikely QuestionBank
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Mole. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
Q1
A gas cylinder contains 12 x1024 molecules of oxygen gas
If Avogadro's number is 6x1023,Calculate:
(i) the mass of oxygen present in the cylinder
(ii) the volume of oxygen at S.T.P. present in the cylinder.[O=16]
solutions
If Avogadro's number is 6x1023,Calculate:
(i) the mass of oxygen present in the cylinder
(ii) the volume of oxygen at S.T.P. present in the cylinder.[O=16]
solutions

Q2
A gaseous hydrocarbon contains 82.76% of carbon.Given that its vapour density is 29,find its
molecular
formula.[C=12,H=1]
solutions
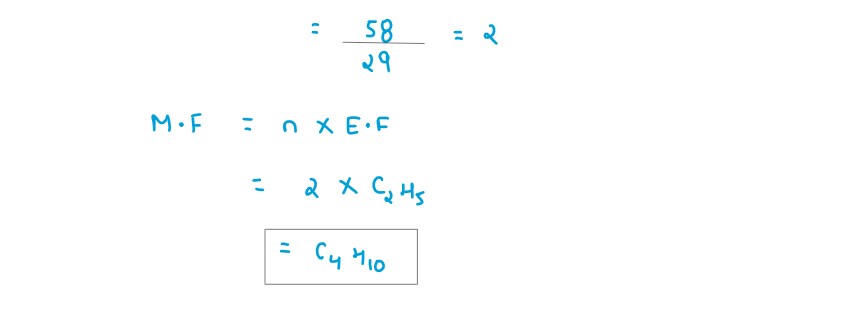
solutions

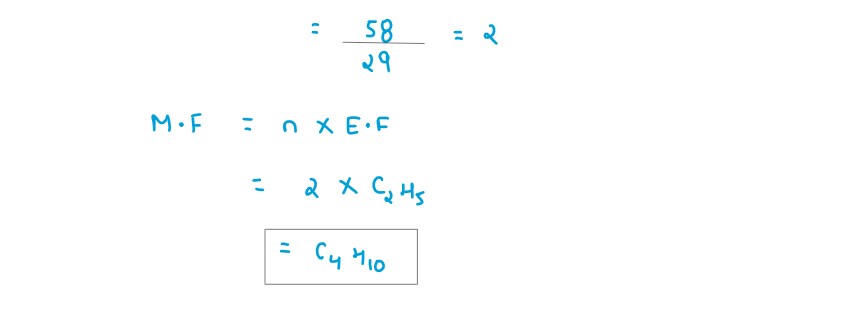
Q3
The equation 4NH3 + 5O2 --> 4NO + 6H2O, represents the catalytic
oxidation of ammonia.If 100 cm3 of ammonia is used the volume of oxygen required to
oxidise
the ammonia completely
solutions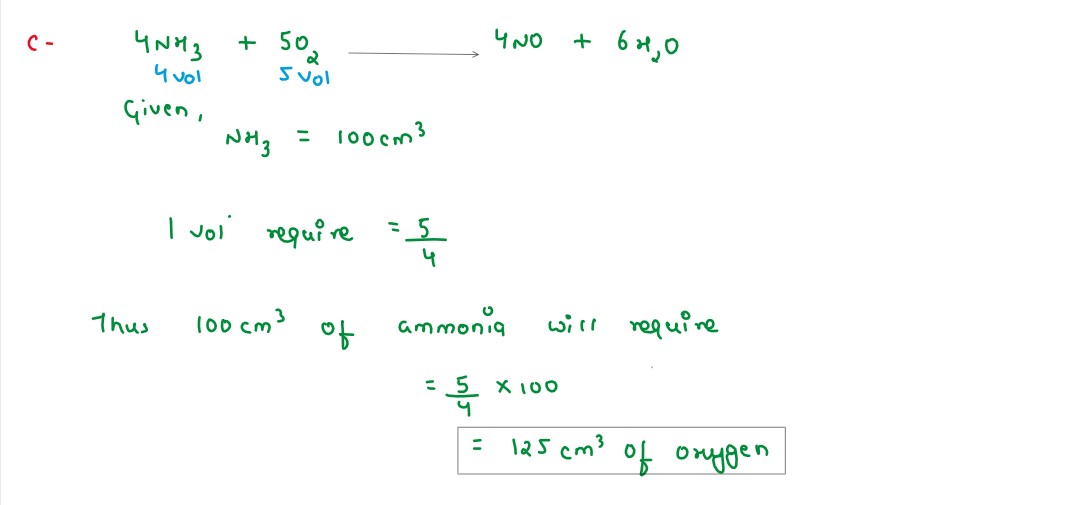
solutions
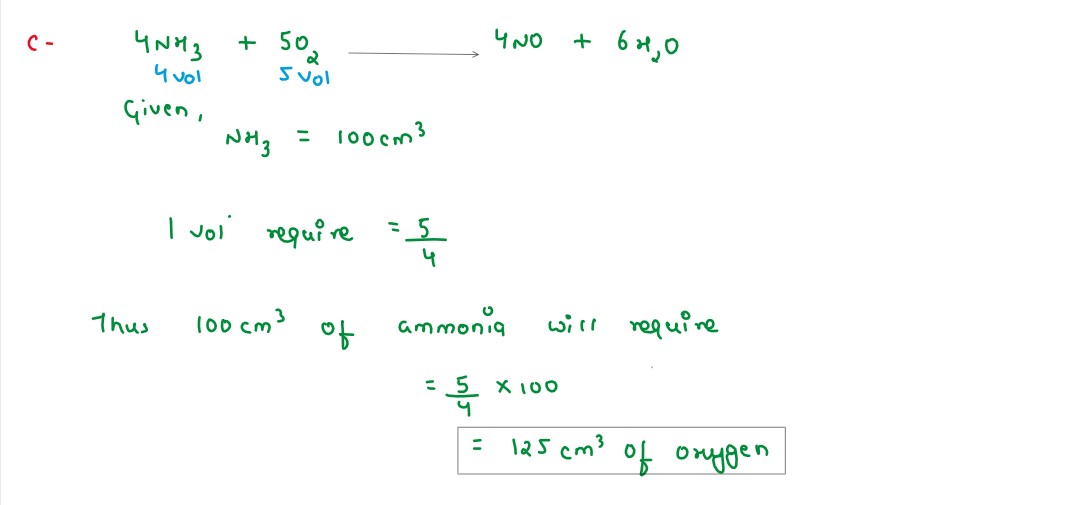
Q4
State the volume occupied by 40 gm of methane at STP,if its vapour density (V.D.) is 8
solutions
solutions

Q5
Calculate the number of moles present in 160gm of NaOH.
[Atomic Mass:Na=23,H=1,O=16]
solutions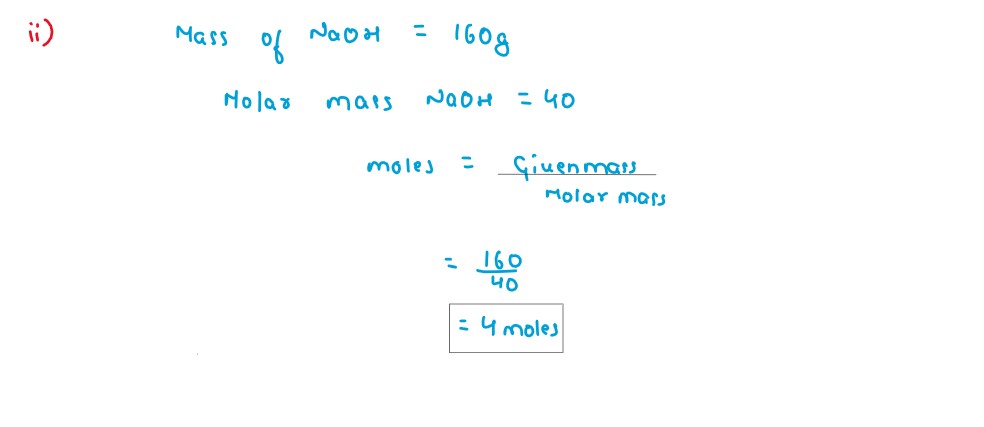
[Atomic Mass:Na=23,H=1,O=16]
solutions
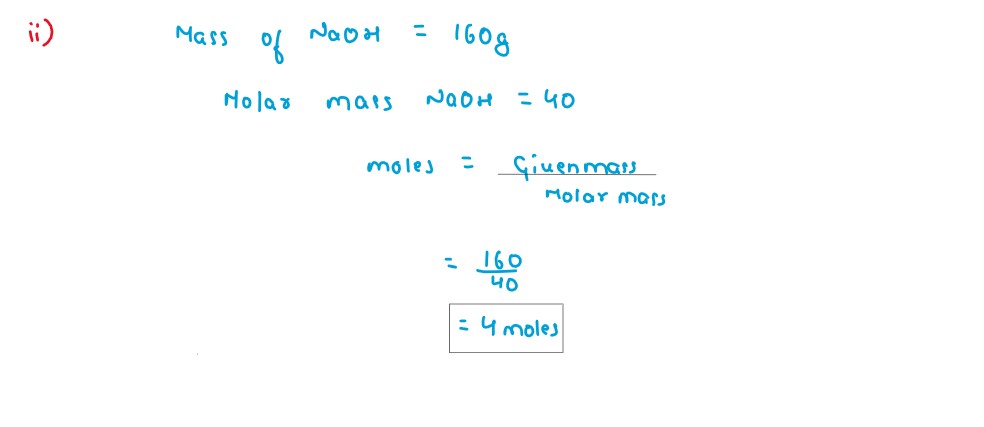

Add a comment