Class 10 Chemistry ICSE Periodic Properties Predicted Questions
Here we provide Class 10 chemistry important notes,board questions and predicted questions with Answers for chapter Periodic Properties. These important notes,board questions and predicted questions are based on ICSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Board examinations.
class 10 ICSE Periodic Properties Mostlikely-QuestionBank
Periodic Properties Mostlikely-QuestionBank
Q1
Name
(a) An alkali metal in. 3 and halogen in period 2
(b) The noble gas with 3 shells?
(c) The non metals present in. 2 and metals in period 3
(d) The element of period 3 with valency 4
(e) The element in period 3 which does not form oxide
(f) The element of lower nuclear charge out of Be and Mg
solutions
(a) An alkali metal in. 3 and halogen in period 2
(b) The noble gas with 3 shells?
(c) The non metals present in. 2 and metals in period 3
(d) The element of period 3 with valency 4
(e) The element in period 3 which does not form oxide
(f) The element of lower nuclear charge out of Be and Mg
solutions

Q2
Match the atomic number 19,5,8,4 and 2 with each of the following:
(i) A metal of valency one
(ii) A solid non metal of period 3
(iii) A rare gas
(iv) A gaseous element with valency 2
(v) An element of group 2.
solutions
(i) A metal of valency one
(ii) A solid non metal of period 3
(iii) A rare gas
(iv) A gaseous element with valency 2
(v) An element of group 2.
solutions

Q3
Arrange:
(i) Be, li, C, B, N, O, F (in increasing metallic character)
(ii) Si, Na, Al, Mg, Cl, P, S (in decreasing non-metallic character)
solutions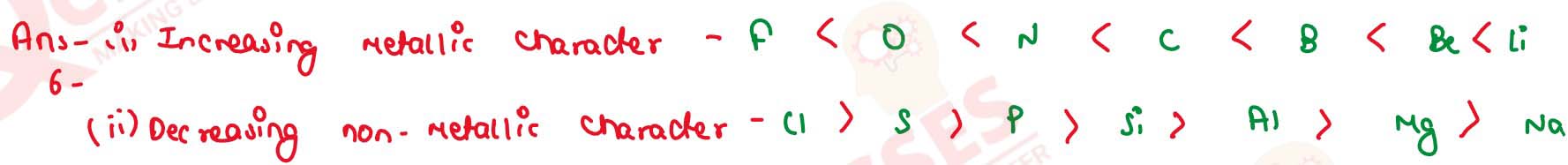
(i) Be, li, C, B, N, O, F (in increasing metallic character)
(ii) Si, Na, Al, Mg, Cl, P, S (in decreasing non-metallic character)
solutions
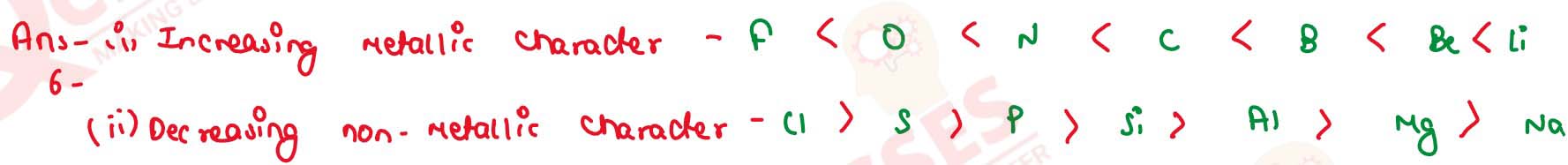
Q4
An element X belongs to 3rd period and 17th group state:
(i) Number of valence electrons in it
(ii) Name of the element
(iii) Name the family to which it belongs
(iv) Write the formula of the compound formed when X reacts with 2713Y
solutions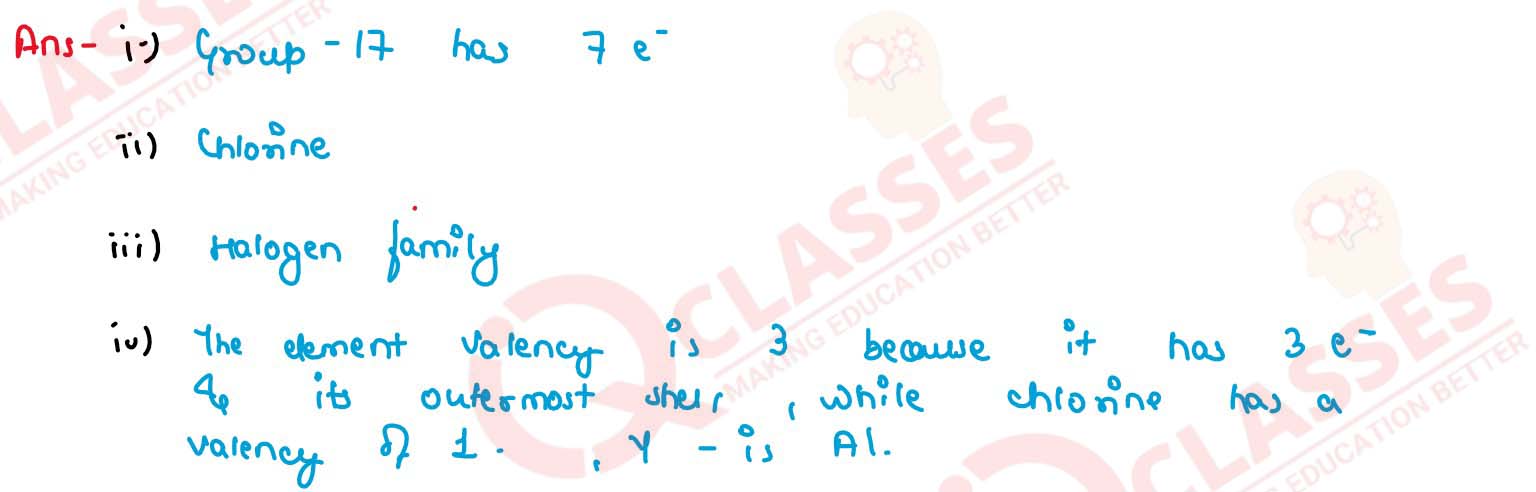
(i) Number of valence electrons in it
(ii) Name of the element
(iii) Name the family to which it belongs
(iv) Write the formula of the compound formed when X reacts with 2713Y
solutions

Q5
(a) Define the term 'electron affinity'. State its unit.
(b) Arrange the elements of second period in increasing order of their electron affinity. Name the elements which do not follow the trend in this.
solutions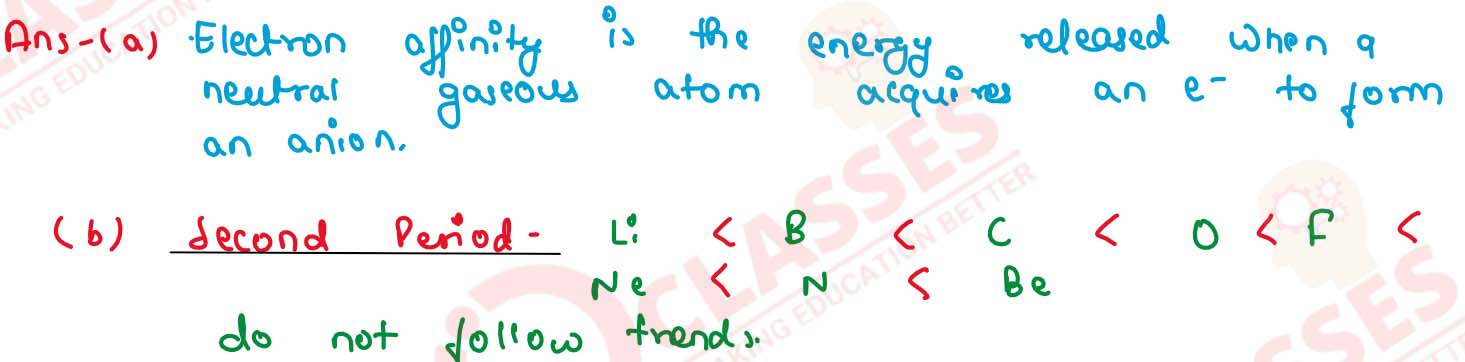
(b) Arrange the elements of second period in increasing order of their electron affinity. Name the elements which do not follow the trend in this.
solutions

Q6
(a) Choose the correct answer from the option an element in period 3 whose electron affinity is
zero:
A. Neon
B. Sulphur
C. Sodium
D. Argon
(b) Give reason:
(i) Analyzation potential of the element increases across a period from left to right.
(ii) Alkali metals are good reducing agents.
(c) There are 3 elements E,F,G with atomic number 19, 8 and 17 respectively-
Classify the above element as metals and non metals.
(d) Name: A metal present in period 3, group 1 of the periodic table.
solutions
A. Neon
B. Sulphur
C. Sodium
D. Argon
(b) Give reason:
(i) Analyzation potential of the element increases across a period from left to right.
(ii) Alkali metals are good reducing agents.
(c) There are 3 elements E,F,G with atomic number 19, 8 and 17 respectively-
Classify the above element as metals and non metals.
(d) Name: A metal present in period 3, group 1 of the periodic table.
solutions

Q7
Choose the word or phrase from the brackets which
correctly completes each of the following statements :-
(i) The element below sodium in the same group would be expected to have a ......... (lower/higher) electro-negativity than sodium and the element above chlorine would be expected to have 4 ............. (lower/higher) ionization potential than chlorine.
(ii) On moving from left to right in a given period, the number of shells ............. (remains the same/ increases decreases).
(iii) On moving down a group, the number of valence electrons ............. (remains the same/increases/ decreases).
(iv) Metals are good ........... (oxidising agent/ reducing agent) because they are electron ............ (acceptors/donors).
solutions
(i) The element below sodium in the same group would be expected to have a ......... (lower/higher) electro-negativity than sodium and the element above chlorine would be expected to have 4 ............. (lower/higher) ionization potential than chlorine.
(ii) On moving from left to right in a given period, the number of shells ............. (remains the same/ increases decreases).
(iii) On moving down a group, the number of valence electrons ............. (remains the same/increases/ decreases).
(iv) Metals are good ........... (oxidising agent/ reducing agent) because they are electron ............ (acceptors/donors).
solutions

Q8
(a) Metals are good .......... (oxidising/reducing agent) because they are electron ..........
(acceptor/doner).
(b) An element with atomic number 19 is most likely combine chemically with the elements whose atomic number is :
(i) 17. (ii) 1 (iii) 28 (iv) 20
(c) Rewrite the following sentences by using the correct symbol > (greater than) or < (less than) in the blanks given :
(i) The ionization potential of Potassium is...........that of Sodium.
(ii) The electronegativity of Iodine iS ...... that of Chlorine.
(d) Fill in the blanks by selecting the correct word from the brackets :
(i) If an element has a low ionization energy then it is likely to be ......... (metallic/non metallic).
(ii) If an element has seven electrons in its outermost shell then it is likely to have the .......... (largest/smallest) atomic size among all the elements in the same period.
solutions
(b) An element with atomic number 19 is most likely combine chemically with the elements whose atomic number is :
(i) 17. (ii) 1 (iii) 28 (iv) 20
(c) Rewrite the following sentences by using the correct symbol > (greater than) or < (less than) in the blanks given :
(i) The ionization potential of Potassium is...........that of Sodium.
(ii) The electronegativity of Iodine iS ...... that of Chlorine.
(d) Fill in the blanks by selecting the correct word from the brackets :
(i) If an element has a low ionization energy then it is likely to be ......... (metallic/non metallic).
(ii) If an element has seven electrons in its outermost shell then it is likely to have the .......... (largest/smallest) atomic size among all the elements in the same period.
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment